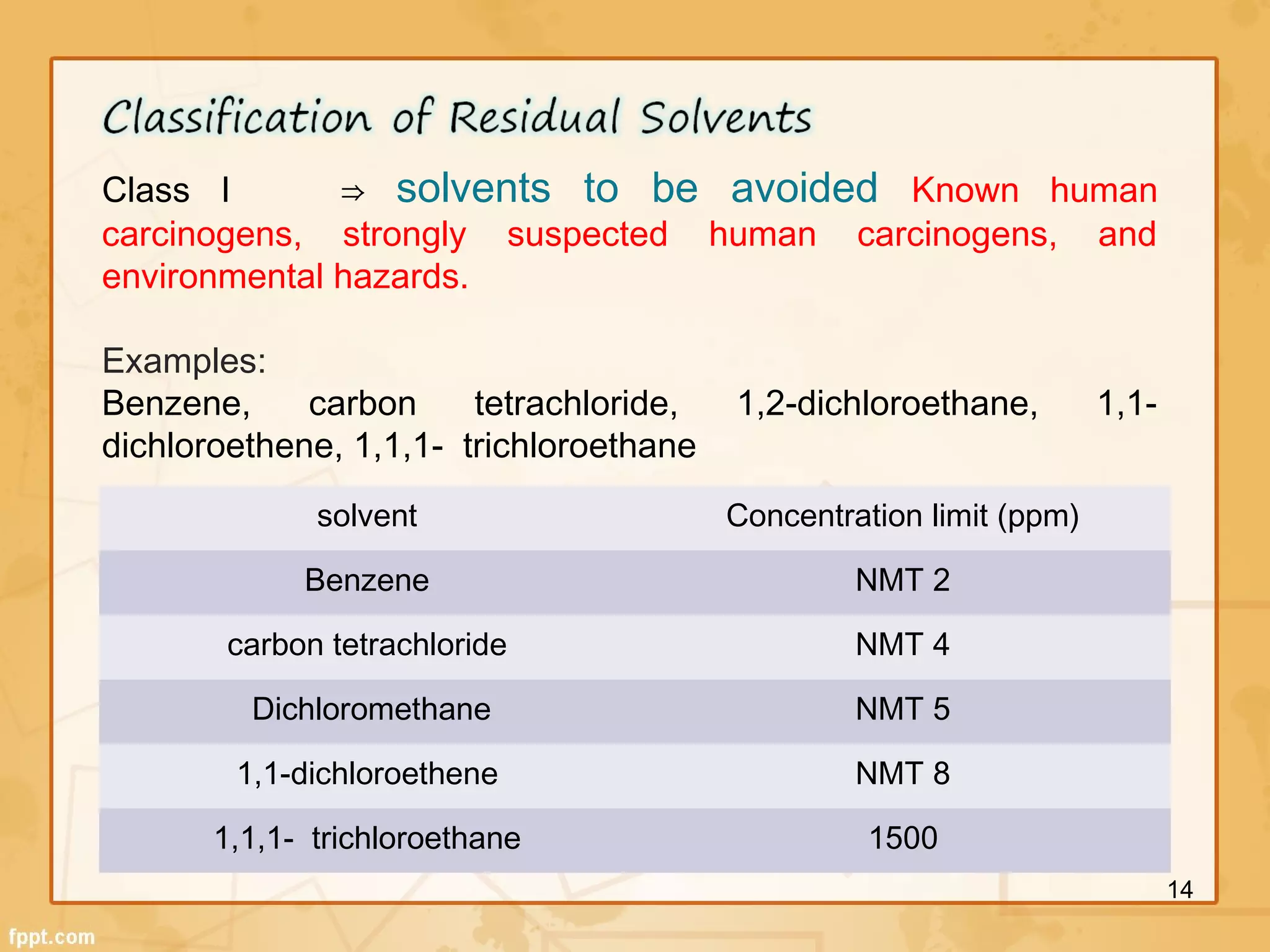
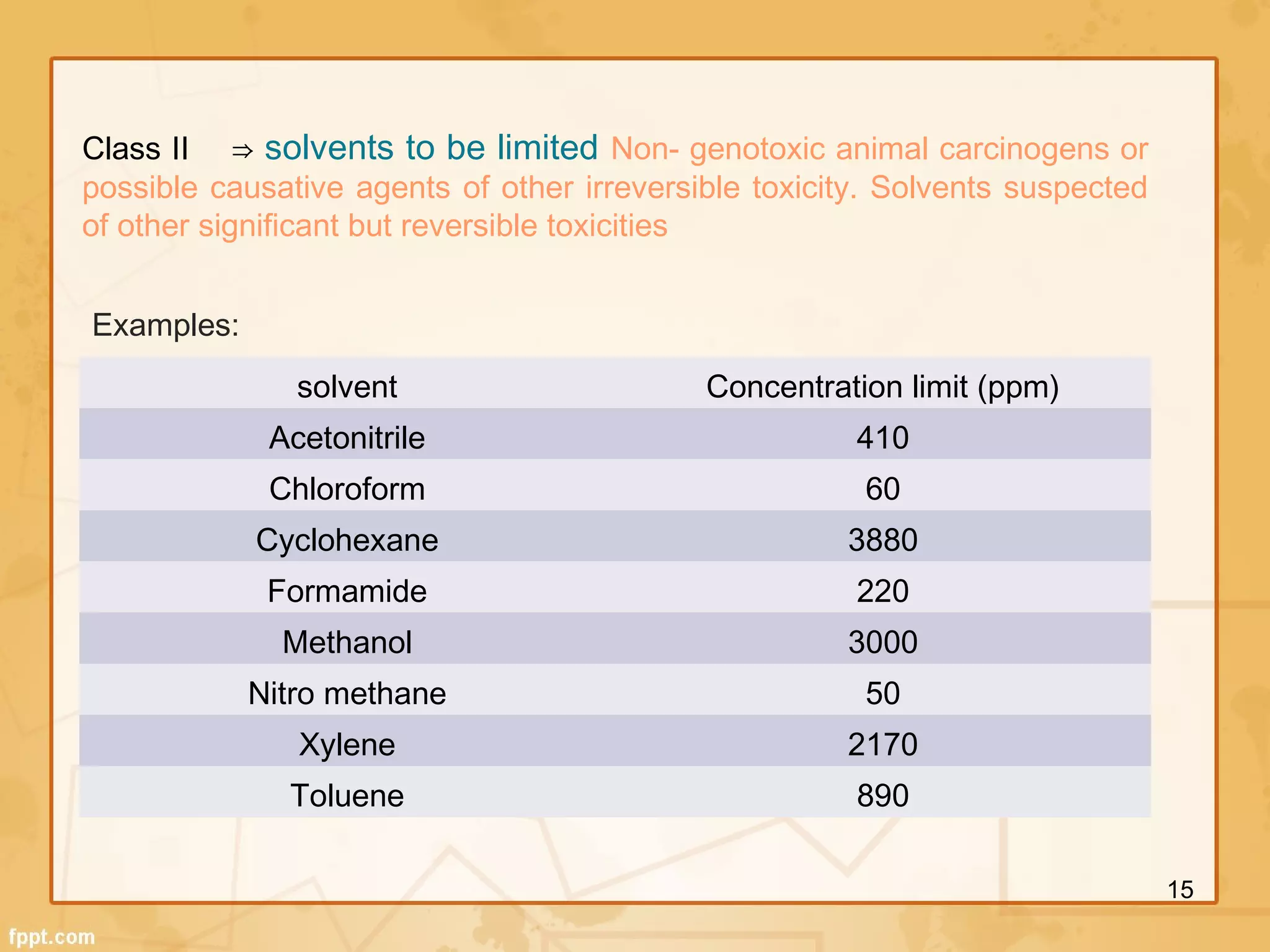
The ICH Q3 guidelines address chemistry and safety aspects of impurities in pharmaceuticals. They define thresholds for reporting, identifying, and qualifying impurities in active pharmaceutical ingredients and finished drug products. Impurities include organic and inorganic compounds arising from manufacturing as well as residual solvents. The guidelines recommend identifying impurities above thresholds and qualifying those above identification levels through genotoxicity testing and evaluating risk to ensure product safety.