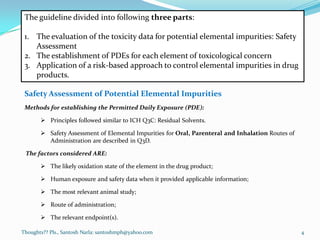
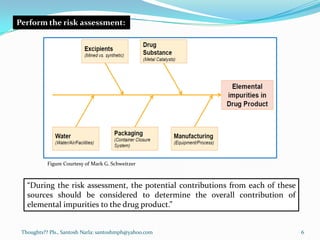
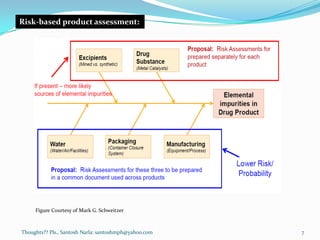
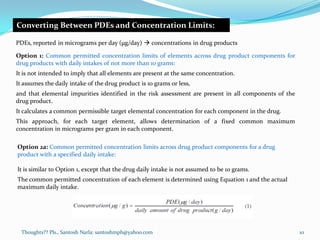
The document discusses guidelines for controlling elemental impurities in pharmaceutical products according to ICH Q3D. It provides information on:
- Common sources of elemental impurities in drug products
- Classification of elements into categories based on their toxicity and likelihood of occurrence
- Methods for establishing permitted daily exposures (PDEs) for elements
- A risk-based approach to assessing and controlling elemental impurities that includes identifying potential sources, evaluating levels compared to PDEs, and documenting control plans
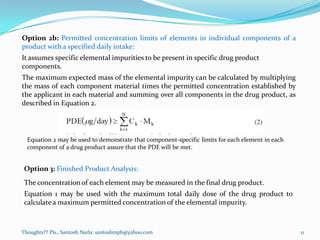
- Options for converting PDEs into concentration limits in drug products or components
The guidelines aim to replace qualitative heavy metal limits with quantitative control of specific elemental impurities shown to have no therapeutic benefit. Manufacturers must