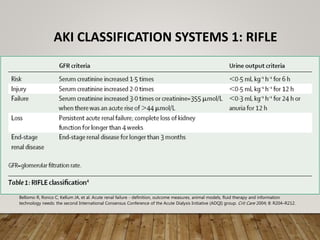
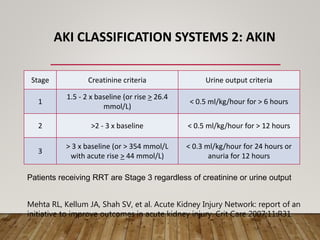
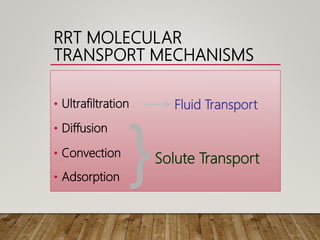
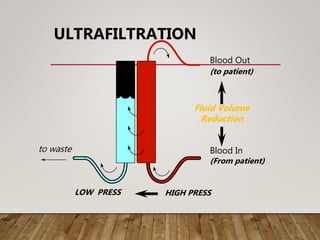
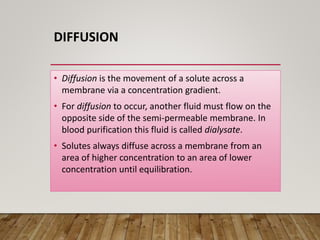
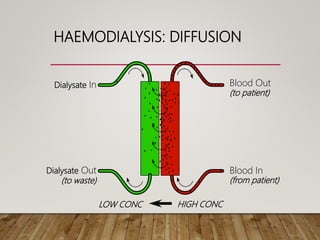
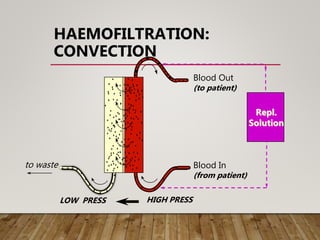
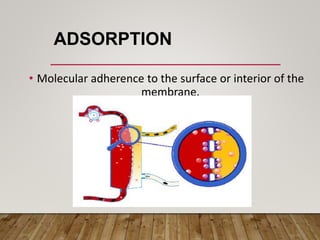
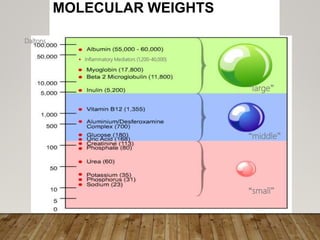
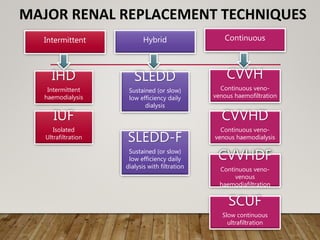
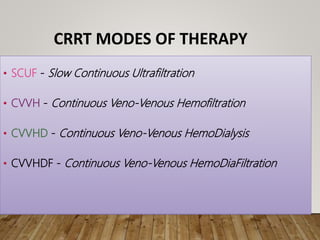
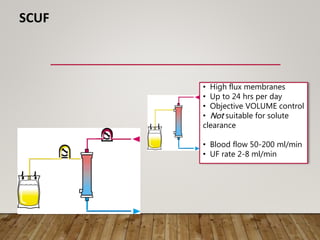
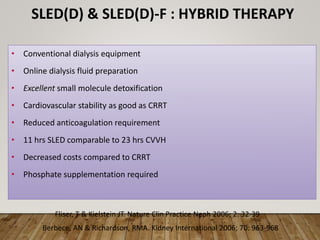
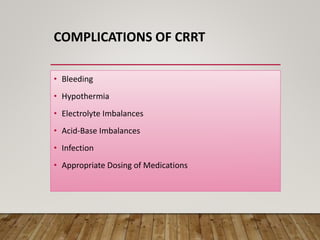
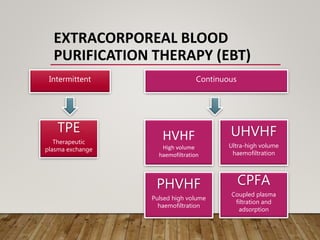
- Renal replacement therapies are important in critical care for managing complications of renal failure such as fluid, electrolyte and acid-base imbalances. There are many questions around optimal therapy including timing, dose and modality.
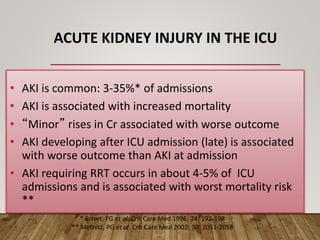
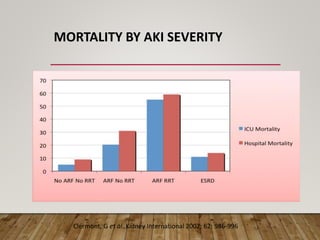
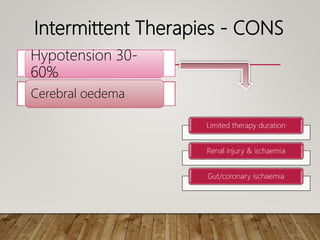
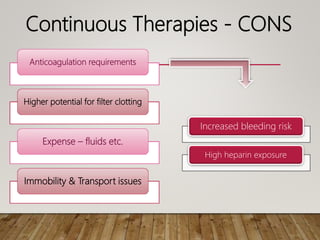
- Acute kidney injury is common in the ICU and associated with worse outcomes. Continuous renal replacement therapies may provide more stable volume and chemistry control compared to intermittent therapies.
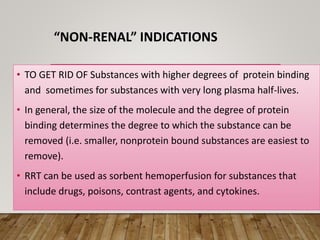
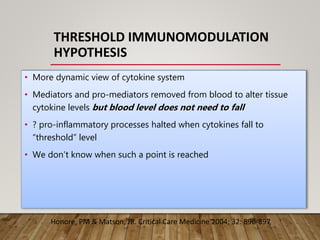
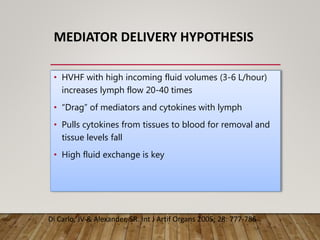
- High volume hemofiltration shows promise for removing inflammatory mediators in sepsis but optimal dose is still unclear. Renal replacement therapies have an important role beyond renal support as blood purification techniques.