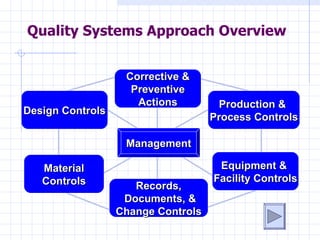
Quality Systems Approach Overview
•Download as PPT, PDF•
28 likes•11,715 views
This document provides an overview of a quality systems approach. It discusses key elements like design controls, production and process controls, corrective and preventive actions, management reviews, and continuous improvement. The quality system aims to design quality in from the beginning, execute according to design, and monitor and control through quality assurance. It also discusses applying this approach to pharmaceutical development and manufacturing through concepts like an integrated validation master plan and quality evaluations.
Report
Share
Report
Share
Recommended
Role of quality systems and audits in pharmaceutical manufacturing environment

By regulation, appropriate practice, and common sense, quality assurance (QA) is a critical function in the pharmaceutical manufacturing environment. The need for an independent unit to audit and comment on the appropriate application of standard operating procedures, master batch records, procedures approved in product applications, and the proper functioning of the quality control (QC) unit is paramount.
This helps assure that products are manufactured reliably, with adherence to approved specifications, and that current good manufacturing practices (cGMP) are maintained in conformance to regulation, both in the facility in general and the microenvironment of each product ’s manufacturing sequence.
Quality System and Audit.pptx

This presentation describes outlines and discusses the regulations
applicable to the QA function and unit, structure, function and
application of the unit in the pharmaceutical manufacturing
environment. In addition, it discusses additional quality – related
responsibilities that may result when manufactures move toward a
quality system approach to quality that incorporates current quality
system models to further improve quality and harmonize with inter-
national quality requirements.
Auditing of vendors and production department

Auditing of vendors and production department quality assurance (QA) M.pharm 2 SEM 1 year of audit and regulatory compliance of
Role of quality system and audits in pharmamaceutical

Introduction
cGMP Regulations
Quality Assurance Function
Quality Systems Approach
Management Responsibilities
Resources
Manufacturing Operations
Evaluation Activities
Transitioning to Quality Systems Approach
Audit Checklist for Drug Industry
Six system inspection model

six system inspection model ( quality management system) for pharmaceutical industry
Recommended
Role of quality systems and audits in pharmaceutical manufacturing environment

By regulation, appropriate practice, and common sense, quality assurance (QA) is a critical function in the pharmaceutical manufacturing environment. The need for an independent unit to audit and comment on the appropriate application of standard operating procedures, master batch records, procedures approved in product applications, and the proper functioning of the quality control (QC) unit is paramount.
This helps assure that products are manufactured reliably, with adherence to approved specifications, and that current good manufacturing practices (cGMP) are maintained in conformance to regulation, both in the facility in general and the microenvironment of each product ’s manufacturing sequence.
Quality System and Audit.pptx

This presentation describes outlines and discusses the regulations
applicable to the QA function and unit, structure, function and
application of the unit in the pharmaceutical manufacturing
environment. In addition, it discusses additional quality – related
responsibilities that may result when manufactures move toward a
quality system approach to quality that incorporates current quality
system models to further improve quality and harmonize with inter-
national quality requirements.
Auditing of vendors and production department

Auditing of vendors and production department quality assurance (QA) M.pharm 2 SEM 1 year of audit and regulatory compliance of
Role of quality system and audits in pharmamaceutical

Introduction
cGMP Regulations
Quality Assurance Function
Quality Systems Approach
Management Responsibilities
Resources
Manufacturing Operations
Evaluation Activities
Transitioning to Quality Systems Approach
Audit Checklist for Drug Industry
Six system inspection model

six system inspection model ( quality management system) for pharmaceutical industry
Auditing of capsule, sterile production and packaging 

Knowledge about auditing of capsule, sterile production and packaging
role of quality system and audit in pharmaceutical manufacturing environment....

M. pharma quality assurance
role of quality system and audit in pharmaceutical manufacturing environment.
topics covered are as follows
cGMP regulation
quality assurance functions
quality system approach
management responsibility
resources
Audit of vendors and Production department 

Detailed about the Production Department and Vendor Audit Which is use as in Pharmaceutical Industries.
Fda initiative on process analytical technology

Process Analytical Technology is used to describe optimal applications of process analytical chemistry tools, feedback process control strategies, information management tools and process / product optimization strategies to the manufacture of pharmaceuticals.
CHAPTER-1 DEFICIENCIES.pdf

As the audit proceeds, there might arise some situations where the facts indicate there is a failure, either partially or wholly, of the quality management system, such a situation is called nonconformity/ deficiencies”.
Quality Audit in pharmaceutical industry

It deals with the understanding and process for auditing
pharmaceutical industries. This covers the methodology involved in auditing process of different in pharmaceutical industries.
Air circulation maintenance industry for sterile and non sterile area

How do we maintain air circulation in sterile and non sterile area - Briefly Described
Pat process analytical technique

process analytical technique , pharmacy , quality assurance technique , QAT, Pharmaceutical quality, quality control.
Cleaning validation

It is process of “Establishing documentary evidence that provide a high degree of assurance that a specific process will consistently produce a product meeting its predetermined specifications and quality attributes”.
In the pharmaceutical industry, it is very important that in addition to final testing and compliance of products, it is also assured that the process will consistently produce the expected results.
Validation is action of proving in accordance with the principles of good manufacturing practices, that any procedure, process, equipment, material, activity or system actually leads to expected results.
Cleaning validation is documented evidence with a high degree assurance that one can consistently clean a system or a piece of equipment to predetermined and acceptable limits.
The primary regulatory concern driving the need for cleaning validation is cross contamination of the desired drug substance either by other API from previous batch runs or by residues from the cleaning agents used.
The prime purpose of validating a cleaning process is to ensure compliance with federal and other standard regulations
1. Cross contamination with active ingredients
Contamination of one batch of product with significant levels of residual active ingredients from previous batch cannot be tolerated.
In addition to the obvious problems posed by subjecting consumers or patients to unintended contaminants, potential clinically significant synergistic interactions between pharmacologically active chemicals are a real concern.
2. Contamination with unintended materials or compounds
While inert ingredients used in drug products are generally recognized as safe for human consumption, the routine use, maintenance and cleaning of equipment's provide the potential contamination with such items as equipment parts, lubricants and chemical cleaning agents3. Microbiological contamination
Maintenance , cleaning and storage conditions may provide adventitious microorganisms with the opportunity to proliferate within the processing equipment.
AUDITING OF QUALITY ASSURANCE AND ENGINEERING DEPARTMENT.pptx

This Slideshare Contain a Brief information about the How Auditing Of QA Department is considered and followed in the Industry to get . Desired Quality product throughout the all production step and in the batch .
Objective importance and Advantages of QA Auditing are explained here. In this slide for giving out and detailed study About it .
Requalification

In this slide contains introduction, qualification, preventive maintenance, requalification method.
Presented by: Malarvannan M (Department of pharmaceutical analysis).RIPER, anantapur
Audits and Regulatory Compliance

objective, management, responsibility, planning process, information gathering types, deficiencies. Administration.
USFDA guidelines on process validation a life cycle approach

validation on lifecycle approach as per as the USFDA
2015 Key Data - 4th Energy Wave Fuel Cell and Hydrogen Annual Review, 2016

Key data from the 4th Energy Wave Fuel Cell and Hydrogen Annual Review 2016. Report available at www.4thenergywave.com.
More Related Content
What's hot
Auditing of capsule, sterile production and packaging 

Knowledge about auditing of capsule, sterile production and packaging
role of quality system and audit in pharmaceutical manufacturing environment....

M. pharma quality assurance
role of quality system and audit in pharmaceutical manufacturing environment.
topics covered are as follows
cGMP regulation
quality assurance functions
quality system approach
management responsibility
resources
Audit of vendors and Production department 

Detailed about the Production Department and Vendor Audit Which is use as in Pharmaceutical Industries.
Fda initiative on process analytical technology

Process Analytical Technology is used to describe optimal applications of process analytical chemistry tools, feedback process control strategies, information management tools and process / product optimization strategies to the manufacture of pharmaceuticals.
CHAPTER-1 DEFICIENCIES.pdf

As the audit proceeds, there might arise some situations where the facts indicate there is a failure, either partially or wholly, of the quality management system, such a situation is called nonconformity/ deficiencies”.
Quality Audit in pharmaceutical industry

It deals with the understanding and process for auditing
pharmaceutical industries. This covers the methodology involved in auditing process of different in pharmaceutical industries.
Air circulation maintenance industry for sterile and non sterile area

How do we maintain air circulation in sterile and non sterile area - Briefly Described
Pat process analytical technique

process analytical technique , pharmacy , quality assurance technique , QAT, Pharmaceutical quality, quality control.
Cleaning validation

It is process of “Establishing documentary evidence that provide a high degree of assurance that a specific process will consistently produce a product meeting its predetermined specifications and quality attributes”.
In the pharmaceutical industry, it is very important that in addition to final testing and compliance of products, it is also assured that the process will consistently produce the expected results.
Validation is action of proving in accordance with the principles of good manufacturing practices, that any procedure, process, equipment, material, activity or system actually leads to expected results.
Cleaning validation is documented evidence with a high degree assurance that one can consistently clean a system or a piece of equipment to predetermined and acceptable limits.
The primary regulatory concern driving the need for cleaning validation is cross contamination of the desired drug substance either by other API from previous batch runs or by residues from the cleaning agents used.
The prime purpose of validating a cleaning process is to ensure compliance with federal and other standard regulations
1. Cross contamination with active ingredients
Contamination of one batch of product with significant levels of residual active ingredients from previous batch cannot be tolerated.
In addition to the obvious problems posed by subjecting consumers or patients to unintended contaminants, potential clinically significant synergistic interactions between pharmacologically active chemicals are a real concern.
2. Contamination with unintended materials or compounds
While inert ingredients used in drug products are generally recognized as safe for human consumption, the routine use, maintenance and cleaning of equipment's provide the potential contamination with such items as equipment parts, lubricants and chemical cleaning agents3. Microbiological contamination
Maintenance , cleaning and storage conditions may provide adventitious microorganisms with the opportunity to proliferate within the processing equipment.
AUDITING OF QUALITY ASSURANCE AND ENGINEERING DEPARTMENT.pptx

This Slideshare Contain a Brief information about the How Auditing Of QA Department is considered and followed in the Industry to get . Desired Quality product throughout the all production step and in the batch .
Objective importance and Advantages of QA Auditing are explained here. In this slide for giving out and detailed study About it .
Requalification

In this slide contains introduction, qualification, preventive maintenance, requalification method.
Presented by: Malarvannan M (Department of pharmaceutical analysis).RIPER, anantapur
Audits and Regulatory Compliance

objective, management, responsibility, planning process, information gathering types, deficiencies. Administration.
USFDA guidelines on process validation a life cycle approach

validation on lifecycle approach as per as the USFDA
What's hot (20)
Auditing of capsule, sterile production and packaging 

Auditing of capsule, sterile production and packaging
role of quality system and audit in pharmaceutical manufacturing environment....

role of quality system and audit in pharmaceutical manufacturing environment....
Air circulation maintenance industry for sterile and non sterile area

Air circulation maintenance industry for sterile and non sterile area
AUDITING OF QUALITY ASSURANCE AND ENGINEERING DEPARTMENT.pptx

AUDITING OF QUALITY ASSURANCE AND ENGINEERING DEPARTMENT.pptx
USFDA guidelines on process validation a life cycle approach

USFDA guidelines on process validation a life cycle approach
Viewers also liked
2015 Key Data - 4th Energy Wave Fuel Cell and Hydrogen Annual Review, 2016

Key data from the 4th Energy Wave Fuel Cell and Hydrogen Annual Review 2016. Report available at www.4thenergywave.com.
Global supply chain in ranbaxy paonta sahib manufacturing facility

This is a project report made by me on the Global Supply Chain Process of Ranbaxy Laboratories Ltd., one of the world's largest Pharmaceutical Manufacturers; and its impact on its Global Business. The related study was done in the year 2011 and report was prepared then.
Computer System Validation is not mere testing

Computer System Validation is one of the critical activities that assures Product Quality and Patients Safety to meet the ultimate goal of the regulatory agencies across the globe. Most traditional SDLC/ testing approaches is not suitable to deploy applications to regulated companies under GxP environment. One should have high technical skills and very good understanding about GxP, Predicate rules, 21 CFR Part-11, Annex-11, GAMP, ISPE and PIC/S to define a process that delivers reliable and quality software to regulated companies. Well-designed Computer System Validation process will assure Product Quality, Patient Safety, Data Security and Data Integrity and eliminates risk of 483 and warning letters.
Computer System Validation Training

Computer System Validation (CSV) is a core requirement for several industries. The aim of Computer System Validation is to ensure, through documentation, that the computer systems function the way they are intended to, consistently, repeatedly and reproducibly, somewhat in the manner expected of scientific experiments. So, the validation, meaning authentication or corroboration, is something that has to be done right from the start, that is, defining the computer system, to their use and going all the way right up to the time the computer system is retired.
Computer System Validation

This presentation describes approaches for software validation used to automate laboratory research procedures, consolidate data collection and analysis and/or run sophisticated QC or manufacturing operations.
Several approaches to software validation exist and may be appropriate for a specific project.
The scope of any validation effort depends upon a number of factors
Size and complexity of the software,
Origin of the software (custom vs. off-the-shelf) and
Whether the functions are critical or non-critical in nature.
By effectively planning the process, validation time and resources can be reduced while meeting regulatory requirements.
Improving Patient Safety and Quality Through Culture, Clinical Analytics, Evi...

According to the Centers of Disease Control (CDC), an estimated 70,000 patients die each year from hospital-associated infections (HAIs): contrast the CDC statistic with the fact that only 35,000 people die each year in the U.S. from motor vehicle accidents. Learn key best practices in patient safety and quality including: patient safety as a team sport, the added challenges of healthcare being the most complex, adaptive system, and how culture, analytics, and content contribute to improve outcomes and lower costs.
Systems approach to management

IT'S A SLIDES ABOUT SYSTEM APPROACH TO MANANAGEMENT ,HAVE A LOOK @ IT ONCE.
Computer System Validation

An overview of Computer System Validation and why it is important when developing systems for pharmaceutical and healthcare industries.
Quality management

Quality Management topic is covered in This PPT. what is quality?, what is Process of Quality management Process, etc.. Present By webneez.com
Total Quality Management (TQM)

This Slideshare presentation is a partial preview of the full business document. To view and download the full document, please go here:
http://flevy.com/browse/business-document/Total-Quality-Management-TQM-152
Total Quality Management (TQM) is a holistic approach to long-term success that views continuous improvement in all aspects of an organization as a process and not as a short-term goal. It aims to radically transform the organization through progressive changes in the attitudes, practices, systems and structures.
By teaching this presentation, employees will understand the importance of making a personal commitment to quality, focus on satisfying both internal and external customer requirements, and working as a team to improve quality.
This training presentation includes quality philosophies from key quality leaders such as W. E. Deming, J. M. Juran and Philip Crosby, and provides a summary of process management, steps for TQM implementation, key tools and techniques for total quality as well as the key business excellence and quality management models.
«Controlled Translation: A new teaching scenario tailor-made for the translat...

«Controlled Translation: A new teaching scenario tailor-made for the translation industry», a paper first presented at the 6th EAMT Workshop, Teaching Machine Translation, noviembre 14-15, 2002. European Association for Machine Translation, pp. 107-116.
What To Say to Build Relationships

A collection of guides for making conversation with the purpose to build relationships: presenting yourself, managing information, making decisions, giving feedback, and others.
Яндекс.Events на Я.Субботнике в Риге, 6 апреля 2013 года

Яндекс.Events?! Краткий рассказ о том, как за 20 лет Яндекс из yet another indexer’а превратился в компанию, где работает почти 4 тысячи человек, половина из которых придумывает и разрабатывает более 40 сервисов, API и технологий. И при этом успевает регулярно рассказывать о них на собственных мероприятиях по всему СНГ, а теперь уже и в Европе. Архивы всех этих презентаций хранятся на сервисе Яндекс.Events.
Formative assessment slides

Some quotes from Dweck, Wiliam and Hattie on the importance of formative assessment.
Viewers also liked (20)
2015 Key Data - 4th Energy Wave Fuel Cell and Hydrogen Annual Review, 2016

2015 Key Data - 4th Energy Wave Fuel Cell and Hydrogen Annual Review, 2016
Global supply chain in ranbaxy paonta sahib manufacturing facility

Global supply chain in ranbaxy paonta sahib manufacturing facility
Improving Patient Safety and Quality Through Culture, Clinical Analytics, Evi...

Improving Patient Safety and Quality Through Culture, Clinical Analytics, Evi...
«Controlled Translation: A new teaching scenario tailor-made for the translat...

«Controlled Translation: A new teaching scenario tailor-made for the translat...
Яндекс.Events на Я.Субботнике в Риге, 6 апреля 2013 года

Яндекс.Events на Я.Субботнике в Риге, 6 апреля 2013 года
Similar to Quality Systems Approach Overview
free training on Quality Management systems in software industry.Iso 9000,ISO...

Concerned with ensuring that the required level of quality is achieved in a software product.
Involves defining appropriate quality standards and procedures and ensuring that these are followed.
Should aim to develop a ‘quality culture’ where quality is seen as everyone’s responsibility.
Computerized System Validation Business Intelligence Solutions

Executive Summary
Regulated pharmaceutical, biotech and medical device companies are challenged to develop manufacturing capabilities quickly and cost-effectively while at the same time safeguarding product quality and patient safety.
Validation has been an essential part of regulated industries for over 20 years, yet as the field has evolved, little has changed in the business, or manual, approach to validation.
Cmmi process overview

CMMi process overview presentations gives brief idea about process implementation in d design house
Quality management

Topics Covered:
1. Quality assurance and standards.
2. Quality planning.
3. Quality control.
PAT and QbD concepts in designing the LiMS and other Electronic systems in La...

tQmlab® is the premier management system for GxP operations and for supporting regulatory submissions. It delivers transformational productivity for QA/QC labs supporting customised workflows for quality control of drugs, stability testing, product release testing and post-release quality testing.
Quality Systems Investigation Technique

An overview of the Quality Systems Investigation Technique used by the Food and Drug Administration (FDA) for on-site inspections. Presented here as a tool for business and industry to use as a guide for internal and external audits and inspections.
Similar to Quality Systems Approach Overview (20)
free training on Quality Management systems in software industry.Iso 9000,ISO...

free training on Quality Management systems in software industry.Iso 9000,ISO...
Computerized System Validation Business Intelligence Solutions

Computerized System Validation Business Intelligence Solutions
PAT and QbD concepts in designing the LiMS and other Electronic systems in La...

PAT and QbD concepts in designing the LiMS and other Electronic systems in La...
Quality assuranceandregulatorycomplianceforpharmaceuticalproduct(4)

Quality assuranceandregulatorycomplianceforpharmaceuticalproduct(4)
More from Mitchell Manning Sr.
Life Coaching Sessions: 2016 at first baptist church robersonville nc

Life coaching sessions taken from the Christian bible in 2016 by Pastor Randal Woodard as edited by Mitchell W. Manning Sr.
2015 world leading life coaches

Life coaching sessions taken from the Christian bible by Pastor Randal Woodard as edited by Mitchell W. Manning Sr.
Train the Trainer for FDA Regulated Industries

A train the trainer guide book for FDA regulated industries.
Social Media Change Agents

An individual or group excercise to explore the core values, work ethic, and thinking of the top 10 social media change agents.
Training and Performance Management Guide

A guide for use by Business and Industry to design, or, upgrade training and performance management documents and processes. This is a companion piece to the Corporate University Catalog on SlideShare.
Time Management: for establishing and controlling your priorities

Time Management: skills, tools, and techniques for taking control of information overload, telephone calls, interruptions, clutter, technology, and work.
Empowering Mega Corp: insights into work/life balance

A collection of slides showing skills, tools, and techniques for achieving balance, synergy, and leverage in your personal and professional life.
This MSPPT presentation is a companion piece for the MSWord presentation by the same name on SlideShare.
Empowering Mega Corp: insights into work/life balance

Skills, tools, and techniques for gaining insight into work/life balance.
Project Management: "made simple" using quick connects

Job aids for initiating, planning, executing, controlling, and closing projects for project team members and project managers.
The project management presentation for the 23rd Annual Southeast ASQ FDC/FDA Conference held February 12, 2010.
Project Management Quick Connects

A brief slide presentation on the technical and behavioral skills for effective project management.
More from Mitchell Manning Sr. (20)
Life Coaching Sessions: 2016 at first baptist church robersonville nc

Life Coaching Sessions: 2016 at first baptist church robersonville nc
Time Management: for establishing and controlling your priorities

Time Management: for establishing and controlling your priorities
Empowering Mega Corp: insights into work/life balance

Empowering Mega Corp: insights into work/life balance
Empowering Mega Corp: insights into work/life balance

Empowering Mega Corp: insights into work/life balance
Project Management: "made simple" using quick connects

Project Management: "made simple" using quick connects
Recently uploaded
Search Disrupted Google’s Leaked Documents Rock the SEO World.pdf

The world of search engine optimization (SEO) is buzzing with discussions after Google confirmed that around 2,500 leaked internal documents related to its Search feature are indeed authentic. The revelation has sparked significant concerns within the SEO community. The leaked documents were initially reported by SEO experts Rand Fishkin and Mike King, igniting widespread analysis and discourse. For More Info:- https://news.arihantwebtech.com/search-disrupted-googles-leaked-documents-rock-the-seo-world/
Tata Group Dials Taiwan for Its Chipmaking Ambition in Gujarat’s Dholera

The Tata Group, a titan of Indian industry, is making waves with its advanced talks with Taiwanese chipmakers Powerchip Semiconductor Manufacturing Corporation (PSMC) and UMC Group. The goal? Establishing a cutting-edge semiconductor fabrication unit (fab) in Dholera, Gujarat. This isn’t just any project; it’s a potential game changer for India’s chipmaking aspirations and a boon for investors seeking promising residential projects in dholera sir.
Visit : https://www.avirahi.com/blog/tata-group-dials-taiwan-for-its-chipmaking-ambition-in-gujarats-dholera/
falcon-invoice-discounting-a-premier-platform-for-investors-in-india

Falcon stands out as a top-tier P2P Invoice Discounting platform in India, bridging esteemed blue-chip companies and eager investors. Our goal is to transform the investment landscape in India by establishing a comprehensive destination for borrowers and investors with diverse profiles and needs, all while minimizing risk. What sets Falcon apart is the elimination of intermediaries such as commercial banks and depository institutions, allowing investors to enjoy higher yields.
Sustainability: Balancing the Environment, Equity & Economy

[Note: This is a partial preview. To download this presentation, visit:
https://www.oeconsulting.com.sg/training-presentations]
Sustainability has become an increasingly critical topic as the world recognizes the need to protect our planet and its resources for future generations. Sustainability means meeting our current needs without compromising the ability of future generations to meet theirs. It involves long-term planning and consideration of the consequences of our actions. The goal is to create strategies that ensure the long-term viability of People, Planet, and Profit.
Leading companies such as Nike, Toyota, and Siemens are prioritizing sustainable innovation in their business models, setting an example for others to follow. In this Sustainability training presentation, you will learn key concepts, principles, and practices of sustainability applicable across industries. This training aims to create awareness and educate employees, senior executives, consultants, and other key stakeholders, including investors, policymakers, and supply chain partners, on the importance and implementation of sustainability.
LEARNING OBJECTIVES
1. Develop a comprehensive understanding of the fundamental principles and concepts that form the foundation of sustainability within corporate environments.
2. Explore the sustainability implementation model, focusing on effective measures and reporting strategies to track and communicate sustainability efforts.
3. Identify and define best practices and critical success factors essential for achieving sustainability goals within organizations.
CONTENTS
1. Introduction and Key Concepts of Sustainability
2. Principles and Practices of Sustainability
3. Measures and Reporting in Sustainability
4. Sustainability Implementation & Best Practices
To download the complete presentation, visit: https://www.oeconsulting.com.sg/training-presentations
Skye Residences | Extended Stay Residences Near Toronto Airport

Experience unparalleled EXTENDED STAY and comfort at Skye Residences located just minutes from Toronto Airport. Discover sophisticated accommodations tailored for discerning travelers.
Website Link :
https://skyeresidences.com/
https://skyeresidences.com/about-us/
https://skyeresidences.com/gallery/
https://skyeresidences.com/rooms/
https://skyeresidences.com/near-by-attractions/
https://skyeresidences.com/commute/
https://skyeresidences.com/contact/
https://skyeresidences.com/queen-suite-with-sofa-bed/
https://skyeresidences.com/queen-suite-with-sofa-bed-and-balcony/
https://skyeresidences.com/queen-suite-with-sofa-bed-accessible/
https://skyeresidences.com/2-bedroom-deluxe-queen-suite-with-sofa-bed/
https://skyeresidences.com/2-bedroom-deluxe-king-queen-suite-with-sofa-bed/
https://skyeresidences.com/2-bedroom-deluxe-queen-suite-with-sofa-bed-accessible/
#Skye Residences Etobicoke, #Skye Residences Near Toronto Airport, #Skye Residences Toronto, #Skye Hotel Toronto, #Skye Hotel Near Toronto Airport, #Hotel Near Toronto Airport, #Near Toronto Airport Accommodation, #Suites Near Toronto Airport, #Etobicoke Suites Near Airport, #Hotel Near Toronto Pearson International Airport, #Toronto Airport Suite Rentals, #Pearson Airport Hotel Suites
5 Things You Need To Know Before Hiring a Videographer

Dive into this presentation to discover the 5 things you need to know before hiring a videographer in Toronto.
ENTREPRENEURSHIP TRAINING.ppt for graduating class (1).ppt

entreprenuership training for graduating students
Putting the SPARK into Virtual Training.pptx

This 60-minute webinar, sponsored by Adobe, was delivered for the Training Mag Network. It explored the five elements of SPARK: Storytelling, Purpose, Action, Relationships, and Kudos. Knowing how to tell a well-structured story is key to building long-term memory. Stating a clear purpose that doesn't take away from the discovery learning process is critical. Ensuring that people move from theory to practical application is imperative. Creating strong social learning is the key to commitment and engagement. Validating and affirming participants' comments is the way to create a positive learning environment.
RMD24 | Retail media: hoe zet je dit in als je geen AH of Unilever bent? Heid...

Grote partijen zijn al een tijdje onderweg met retail media. Ondertussen worden in dit domein ook de kansen zichtbaar voor andere spelers in de markt. Maar met die kansen ontstaan ook vragen: Zelf retail media worden of erop adverteren? In welke fase van de funnel past het en hoe integreer je het in een mediaplan? Wat is nu precies het verschil met marketplaces en Programmatic ads? In dit half uur beslechten we de dilemma's en krijg je antwoorden op wanneer het voor jou tijd is om de volgende stap te zetten.
Premium MEAN Stack Development Solutions for Modern Businesses

Stay ahead of the curve with our premium MEAN Stack Development Solutions. Our expert developers utilize MongoDB, Express.js, AngularJS, and Node.js to create modern and responsive web applications. Trust us for cutting-edge solutions that drive your business growth and success.
Know more: https://www.synapseindia.com/technology/mean-stack-development-company.html
Affordable Stationery Printing Services in Jaipur | Navpack n Print

Looking for professional printing services in Jaipur? Navpack n Print offers high-quality and affordable stationery printing for all your business needs. Stand out with custom stationery designs and fast turnaround times. Contact us today for a quote!
Buy Verified PayPal Account | Buy Google 5 Star Reviews

Buy Verified PayPal Account
Looking to buy verified PayPal accounts? Discover 7 expert tips for safely purchasing a verified PayPal account in 2024. Ensure security and reliability for your transactions.
PayPal Services Features-
🟢 Email Access
🟢 Bank Added
🟢 Card Verified
🟢 Full SSN Provided
🟢 Phone Number Access
🟢 Driving License Copy
🟢 Fasted Delivery
Client Satisfaction is Our First priority. Our services is very appropriate to buy. We assume that the first-rate way to purchase our offerings is to order on the website. If you have any worry in our cooperation usually You can order us on Skype or Telegram.
24/7 Hours Reply/Please Contact
usawebmarketEmail: support@usawebmarket.com
Skype: usawebmarket
Telegram: @usawebmarket
WhatsApp: +1(218) 203-5951
USA WEB MARKET is the Best Verified PayPal, Payoneer, Cash App, Skrill, Neteller, Stripe Account and SEO, SMM Service provider.100%Satisfection granted.100% replacement Granted.
The effects of customers service quality and online reviews on customer loyal...

The effects of customers service quality and online reviews on customer loyal...balatucanapplelovely
Research VAT Registration Outlined In UAE: Benefits and Requirements

Vat Registration is a legal obligation for businesses meeting the threshold requirement, helping companies avoid fines and ramifications. Contact now!
https://viralsocialtrends.com/vat-registration-outlined-in-uae/
ModelingMarketingStrategiesMKS.CollumbiaUniversitypdf

Implicitly or explicitly all competing businesses employ a strategy to select a mix
of marketing resources. Formulating such competitive strategies fundamentally
involves recognizing relationships between elements of the marketing mix (e.g.,
price and product quality), as well as assessing competitive and market conditions
(i.e., industry structure in the language of economics).
Kseniya Leshchenko: Shared development support service model as the way to ma...

Kseniya Leshchenko: Shared development support service model as the way to make small projects with small budgets profitable for the company (UA)
Kyiv PMDay 2024 Summer
Website – www.pmday.org
Youtube – https://www.youtube.com/startuplviv
FB – https://www.facebook.com/pmdayconference
20240425_ TJ Communications Credentials_compressed.pdf

"𝑩𝑬𝑮𝑼𝑵 𝑾𝑰𝑻𝑯 𝑻𝑱 𝑰𝑺 𝑯𝑨𝑳𝑭 𝑫𝑶𝑵𝑬"
𝐓𝐉 𝐂𝐨𝐦𝐬 (𝐓𝐉 𝐂𝐨𝐦𝐦𝐮𝐧𝐢𝐜𝐚𝐭𝐢𝐨𝐧𝐬) is a professional event agency that includes experts in the event-organizing market in Vietnam, Korea, and ASEAN countries. We provide unlimited types of events from Music concerts, Fan meetings, and Culture festivals to Corporate events, Internal company events, Golf tournaments, MICE events, and Exhibitions.
𝐓𝐉 𝐂𝐨𝐦𝐬 provides unlimited package services including such as Event organizing, Event planning, Event production, Manpower, PR marketing, Design 2D/3D, VIP protocols, Interpreter agency, etc.
Sports events - Golf competitions/billiards competitions/company sports events: dynamic and challenging
⭐ 𝐅𝐞𝐚𝐭𝐮𝐫𝐞𝐝 𝐩𝐫𝐨𝐣𝐞𝐜𝐭𝐬:
➢ 2024 BAEKHYUN [Lonsdaleite] IN HO CHI MINH
➢ SUPER JUNIOR-L.S.S. THE SHOW : Th3ee Guys in HO CHI MINH
➢FreenBecky 1st Fan Meeting in Vietnam
➢CHILDREN ART EXHIBITION 2024: BEYOND BARRIERS
➢ WOW K-Music Festival 2023
➢ Winner [CROSS] Tour in HCM
➢ Super Show 9 in HCM with Super Junior
➢ HCMC - Gyeongsangbuk-do Culture and Tourism Festival
➢ Korean Vietnam Partnership - Fair with LG
➢ Korean President visits Samsung Electronics R&D Center
➢ Vietnam Food Expo with Lotte Wellfood
"𝐄𝐯𝐞𝐫𝐲 𝐞𝐯𝐞𝐧𝐭 𝐢𝐬 𝐚 𝐬𝐭𝐨𝐫𝐲, 𝐚 𝐬𝐩𝐞𝐜𝐢𝐚𝐥 𝐣𝐨𝐮𝐫𝐧𝐞𝐲. 𝐖𝐞 𝐚𝐥𝐰𝐚𝐲𝐬 𝐛𝐞𝐥𝐢𝐞𝐯𝐞 𝐭𝐡𝐚𝐭 𝐬𝐡𝐨𝐫𝐭𝐥𝐲 𝐲𝐨𝐮 𝐰𝐢𝐥𝐥 𝐛𝐞 𝐚 𝐩𝐚𝐫𝐭 𝐨𝐟 𝐨𝐮𝐫 𝐬𝐭𝐨𝐫𝐢𝐞𝐬."
Recently uploaded (20)
Search Disrupted Google’s Leaked Documents Rock the SEO World.pdf

Search Disrupted Google’s Leaked Documents Rock the SEO World.pdf
Tata Group Dials Taiwan for Its Chipmaking Ambition in Gujarat’s Dholera

Tata Group Dials Taiwan for Its Chipmaking Ambition in Gujarat’s Dholera
falcon-invoice-discounting-a-premier-platform-for-investors-in-india

falcon-invoice-discounting-a-premier-platform-for-investors-in-india
Sustainability: Balancing the Environment, Equity & Economy

Sustainability: Balancing the Environment, Equity & Economy
Skye Residences | Extended Stay Residences Near Toronto Airport

Skye Residences | Extended Stay Residences Near Toronto Airport
5 Things You Need To Know Before Hiring a Videographer

5 Things You Need To Know Before Hiring a Videographer
ENTREPRENEURSHIP TRAINING.ppt for graduating class (1).ppt

ENTREPRENEURSHIP TRAINING.ppt for graduating class (1).ppt
RMD24 | Retail media: hoe zet je dit in als je geen AH of Unilever bent? Heid...

RMD24 | Retail media: hoe zet je dit in als je geen AH of Unilever bent? Heid...
Premium MEAN Stack Development Solutions for Modern Businesses

Premium MEAN Stack Development Solutions for Modern Businesses
Affordable Stationery Printing Services in Jaipur | Navpack n Print

Affordable Stationery Printing Services in Jaipur | Navpack n Print
Buy Verified PayPal Account | Buy Google 5 Star Reviews

Buy Verified PayPal Account | Buy Google 5 Star Reviews
The effects of customers service quality and online reviews on customer loyal...

The effects of customers service quality and online reviews on customer loyal...
VAT Registration Outlined In UAE: Benefits and Requirements

VAT Registration Outlined In UAE: Benefits and Requirements
ModelingMarketingStrategiesMKS.CollumbiaUniversitypdf

ModelingMarketingStrategiesMKS.CollumbiaUniversitypdf
Kseniya Leshchenko: Shared development support service model as the way to ma...

Kseniya Leshchenko: Shared development support service model as the way to ma...
20240425_ TJ Communications Credentials_compressed.pdf

20240425_ TJ Communications Credentials_compressed.pdf
Quality Systems Approach Overview
- 1. Quality Systems Approach Overview Corrective & Preventive Actions Production & Design Controls Process Controls Management Material Equipment & Controls Facility Controls Records, Documents, & Change Controls
- 2. Quality System Alignment and Integration Continuous Improvement Design Execute Monitor/Control Design – determine what is really important Execute – translate into service and manufacturing Monitor/Control – translate into quality assurance
- 4. Capability and Control Cycle Development History Integrated Validation Master Plan process product systems Begin with the End in Mind 2. Compliant Establish shared standards 3. Capable Process Flow Document and expectations 4. Robust 5. In Control 6. Continuously Improving Technology Transfer Qualification Document with FDA in Mind 2. Clear Execute and monitor with 3. Concise Validation process and product best business practices. 4. Correct 5. Complete Execute, Monitor and Control 6. Confident Technical Evaluations process and product Quality (GMP) Evaluations quality systems Change Control process and product Change Control quality systems Assess results against the standards and practices. Quality Management System
- 8. Scenario Worst Case Most Likely Case Best Case Decision
- 10. Responsibility of Highest Level of Management Establish Quality Policy Ensure that it is followed
- 11. Delegation by Management with Executive Responsibility Establishment of quality objectives Translation of objectives into methods and procedures Implementation of quality system
- 12. How does Management Assure an Effective Quality System? CAPA Management Audits Review
- 13. How to Demonstrate Compliance Procedures ... Verbal Communications Written records and documents
- 14. Establish [21 CFR 820.3(k)] Define Document Implement
- 15. Key Elements of a Quality Manual 1. Generation and maintenance of master production batch records. 2. Generation of routine batch records 3. Generation and maintenance of Standard Operating Procedures 4. Generation and maintenance of preventive maintenance procedures 5. Generation and maintenance of calibration procedures 6. Generation and maintenance of equipment logbooks 7. Generation and maintenance of cleaning procedures 8. Generation and maintenance of deviation/failure reports 9. Generation of rework procedures
- 16. Key Elements of a Quality Manual 1. Training programs and records for all employees 2. In-coming inspection program for raw materials 3. In-process analytical checks during processing 4. Inventory control 6. Validation of equipment/systems/processes 7. Cleaning validation 8. Analytical methods validation 9. Computer/controller validation 10. Validation change control 11. Revalidation program
- 17. Key Elements of a Quality Manual 1. Audit programs - internally and externally 2. Qualification of vendors 3. Quality Control testing (in-process and finished product testing) 4. Complaint handling program 5. Annual product reviews 6. Stability Program 7. Sample retention program 8. Documentation control/storage 9. Labeling and label control 10. Specification development 11. Generation and validation of analytical methods
- 18. Writing and Managing Standard Operating Procedures Controls Approvals Formatting Readability Change History Cross References
- 19. Developing Batch Records CFR 211.188 Prepared for each batch of drug product produced Include complete information relating to production and control of each batch (signed, dated and checked accurate reproduction of master production or control record, documentation of accomplished significant steps in manufacture, processing, packing or holding – dates, equipment and lines, specific identification of components and in-process material, ….)
- 20. Document Management Structure Segregation Documents in Review Documents in Approval Effective Documents Archived Documents Control
- 21. Complaint Management CFR 211.198 Mechanism Designated person in quality group Logged with unique number Sender Detail Sample Appropriate storage conditions Investigation methodology Response Reference book
- 22. Annual Product Review CFR 211.180 (e) FDA Requirement Annual Reports Summary of all findings Sent to FDA by product NDA anniversary date Review of batches Deviations Failures Out of Specifications Stability Profiles Visual verification Retained samples
- 23. Managing Regulatory Training Compliance CFR 211.25 Education, Training, and Experience Enable to perform assigned functions Particular operations performed Current Good Manufacturing Practice Written procedures related to assigned functions Continuing basis Sufficient frequency Documented Follow-up
- 24. How does Management Assure an Effective Quality System? CAPA Management Audits Review
- 25. (Your Organization’s Name) Regulatory Responsibilities Job Title/Employee Quality System 21 CFR Part 211 Subparts A B C D E F G H I J K
- 26. Management Production & Design Controls Process Controls Corrective & Preventive Actions Material Equipment & Controls Facility Controls Records, Documents, & Change Controls Controls
- 27. (Your Organization’s Name) Regulatory Responsibilities • What are the responsibilities? • Where are the gaps? • What are the risks? • What are the consequences? • What are the opportunities? • What are the rewards?
- 28. QUESTIONS
