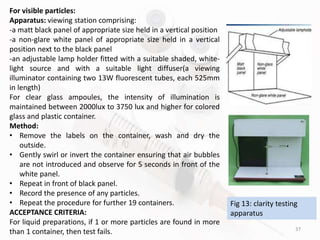
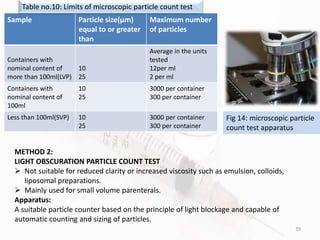
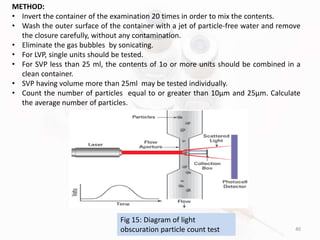
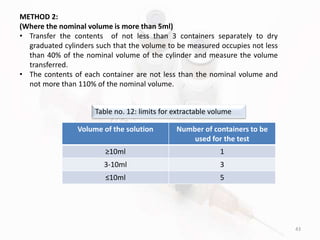
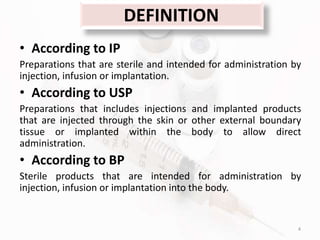
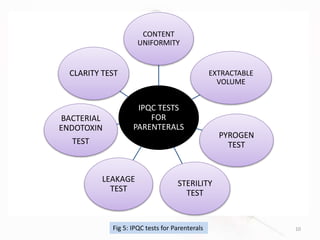
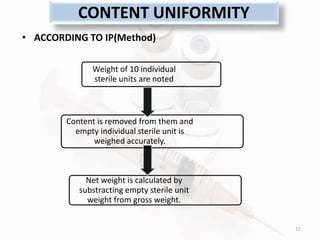
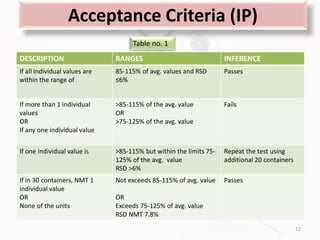
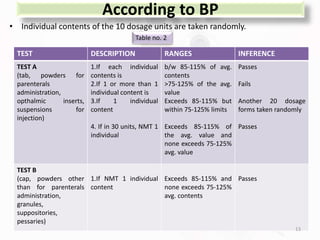
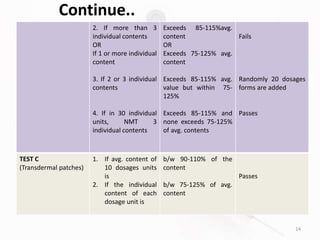
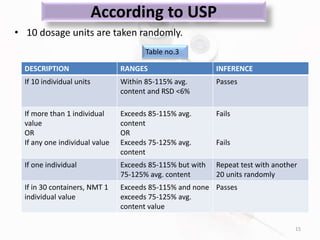
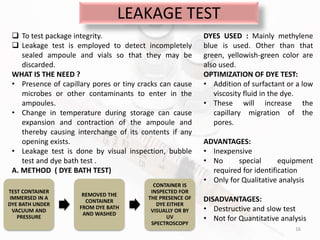
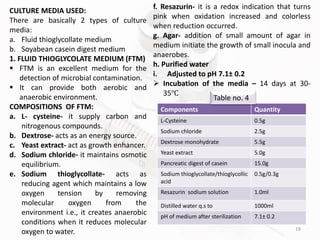
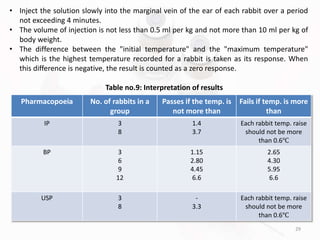
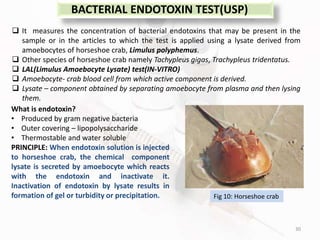
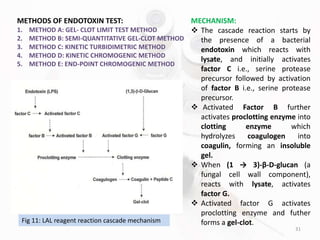
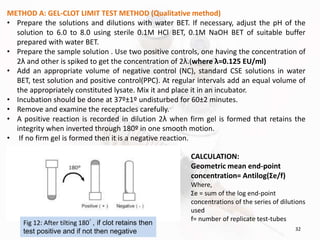
The document discusses in-process and finished product quality control tests for parenterals. It defines parenterals as sterile preparations intended for administration by injection, infusion, or implantation. It describes various types of parenterals including small volume parenterals like ampoules and vials, as well as large volume parenterals. The document then outlines several important in-process quality control tests that are conducted on parenterals to ensure safety, identity, strength, quality and purity. These include tests like content uniformity, leakage, sterility, bacterial endotoxins and clarity. Specific test methods, acceptance criteria and significance are provided for key tests according to compendial standards.


















![33
METHOD B: SEMI-QUANTITATIVE GEL-CLOT METHOD
Preparation of test solutions: Prepare test solutions at concentration of MVD, 0.5
MVD,0.25MVD (Maximum Valid Dilution).
[MVD= Endotoxin limit(EU/mg) X concentration of the test solution(mg/ml)/λ]
Method: Same as Method A
Calculation and interpretation of results
• To calculate the endotoxin concentration in the product, determine for the series of
test solutions the lowest concentration or the highest dilution giving a positive (+)
reaction.
• Multiply this dilution with to obtain the endotoxin concentration of the product.
• For example, if MVD is equal to 8 and the positive reaction was obtained at 0.25
MVD and λ was equal to 0.125EU/ml, then endotoxin concentration in the test will
be, 8x 0.25x 0.125=0.25 EU/ml.
• If none of the dilutions of the series gives a positive reaction, the endotoxin
concentration will be less than the value obtained by multiplying the lowest diltution
factor with λ.
• If all the dilutions of the series give a positive reaction, the endotoxin concentration
will be more than the value obtained by multiplying the highest dilution factor with
λ.](https://image.slidesharecdn.com/ipqctestsforparenterals-200429070353/85/IPQC-tests-for-Parenterals-33-320.jpg)