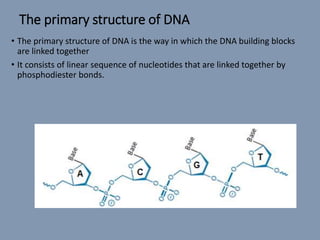
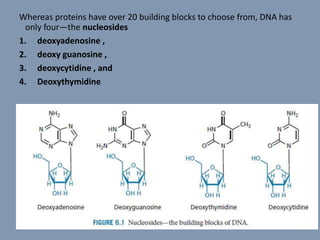
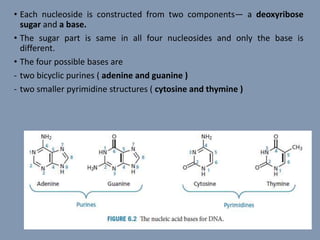
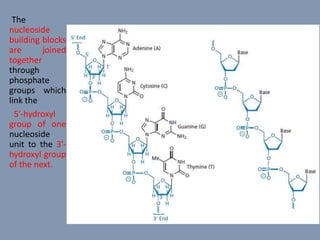
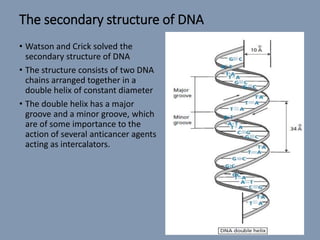
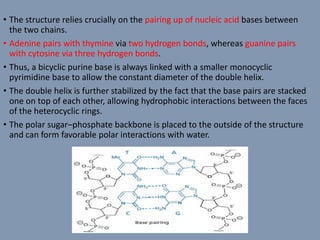
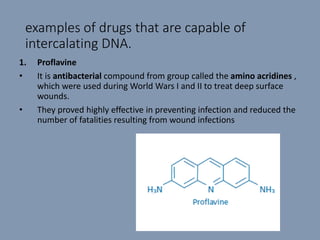
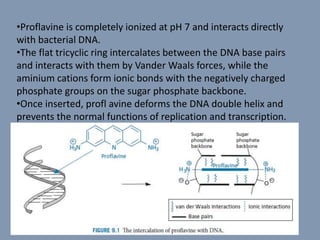
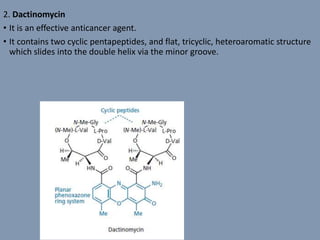
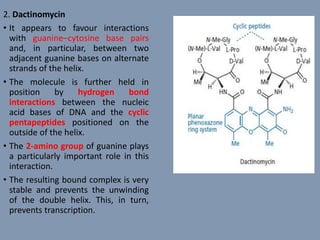
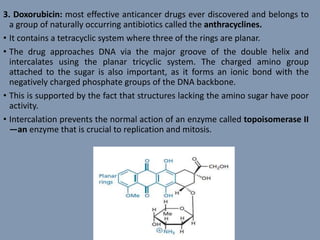
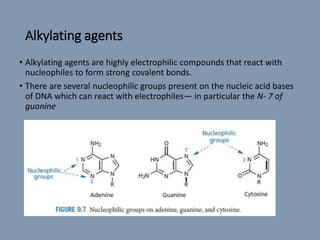
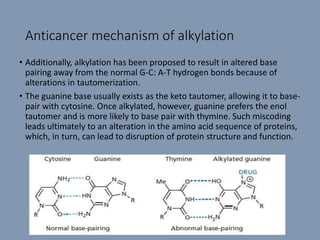
The document discusses nucleic acids, focusing on the structure and function of DNA and RNA, including their primary, secondary, and tertiary structures. It highlights the importance of nucleic acids as drug targets, particularly in anticancer and antibacterial therapy, and categorizes the types of drugs that interact with DNA, such as intercalating agents and alkylating agents. The document provides examples of specific drugs and their mechanisms of action against DNA, emphasizing how they interfere with replication and transcription.