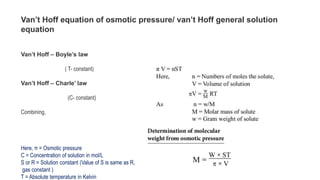
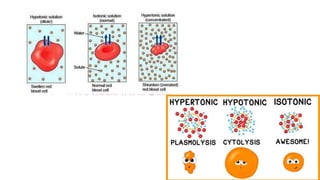
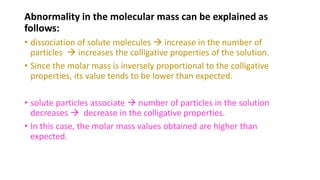
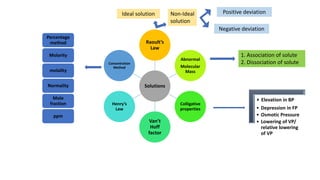
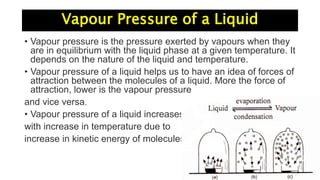
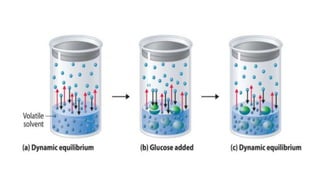
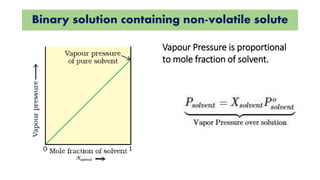
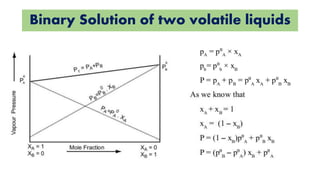
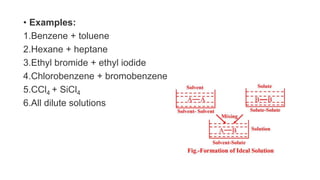
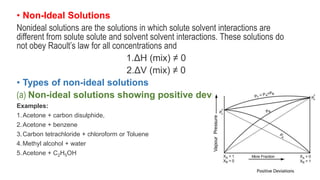
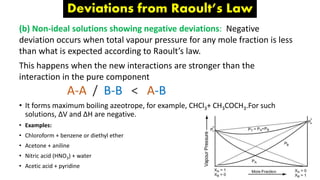
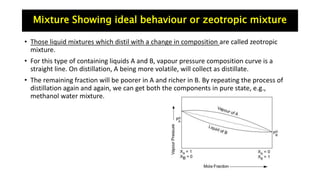
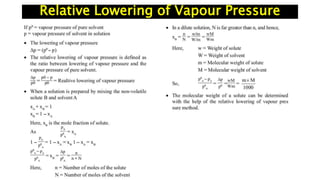
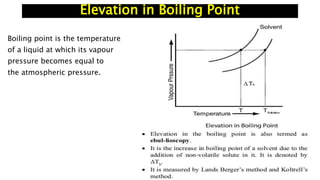
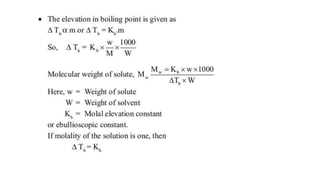
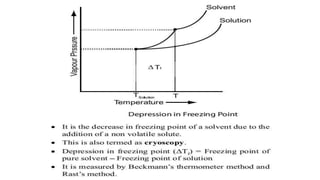
The document discusses various topics related to solutions and colligative properties. It defines key terms like solute, solvent, concentration methods. It explains concepts such as Raoult's law, deviations from Raoult's law, ideal and non-ideal solutions. It also covers colligative properties including elevation in boiling point, depression in freezing point, osmotic pressure and lowering of vapour pressure. It discusses abnormal molecular masses that can arise from solute dissociation or association and how the van't Hoff factor can explain this.

































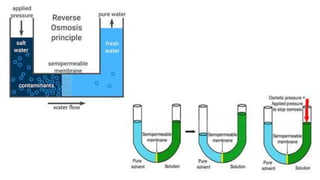
![Osmosis
In osmosis, there is a net flow of solvent molecules from the solvent to the solution
OR
from a less concentrated solution to a more concentrated solution across a
semipermeable membrane (membranes of animal origin, membranes made from
Cu2 [Fe (CN) 6], Ca3 (PO4)2 etc.)
• Osmosis was first observed by
Abbe Nollet in 1748.
Jean-Antoine Nollet (1700-1770) was a French
clergyman and physicist who did a number of
experiments with electricity and discovered osmosis](https://image.slidesharecdn.com/12-solutionscbseclass12-220106065412/85/Solutions-and-Colligative-properties-49-320.jpg)