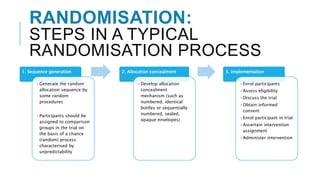
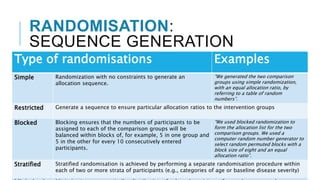
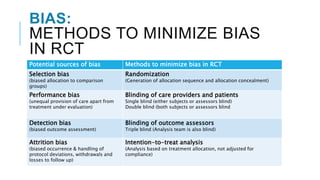
Randomized controlled trials (RCTs) are experimental studies used to test interventions by randomly assigning participants into treatment and control groups. This document outlines key aspects of RCTs including randomization procedures, blinding techniques, and methods to minimize bias. It describes the steps involved in random sequence generation, allocation concealment, and implementing randomization. The document also discusses advantages and disadvantages of RCTs, different trial designs, and reporting standards like CONSORT to improve transparency of RCT results.