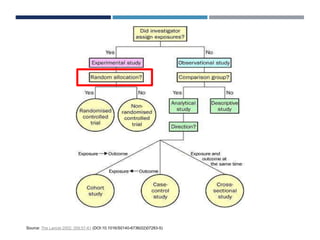
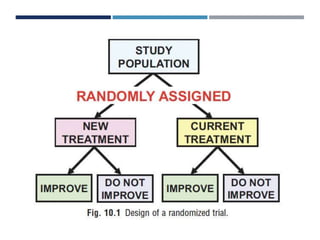
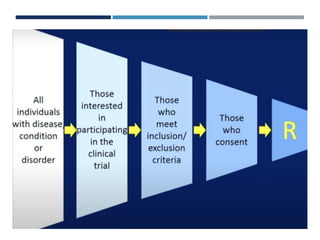
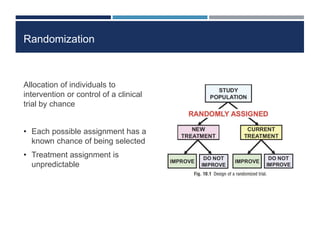
This document discusses randomization in medical research. It defines randomization as the allocation of individuals to intervention or control groups in a clinical trial by chance. Randomization avoids selection bias and balances both known and unknown confounding factors between groups. Participants, communities, or locations can be randomized. Simple, permuted block, stratified, and adaptive randomization methods are described. Threats to randomization include non-adherence to the randomization protocol and lack of treatment equipoise. Maintaining the integrity of randomization is important for establishing causality in medical research.