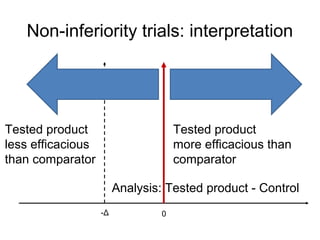
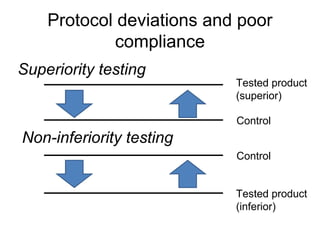
This document discusses non-inferiority clinical trials. It notes that non-inferiority trials are conducted when superiority trials are unethical or impractical. In a non-inferiority trial, the hypothesis is that a new treatment is not clinically inferior to the comparator by more than a pre-specified non-inferiority margin. Protocol deviations and lack of compliance can undermine non-inferiority trials by favoring the conclusion that treatments are non-inferior when they may actually be inferior. It is important that non-inferiority trials adhere closely to protocols and measure compliance to avoid invalid conclusions.