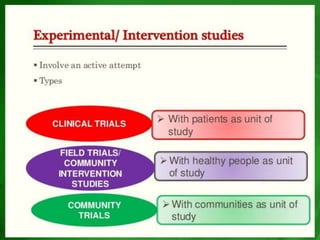
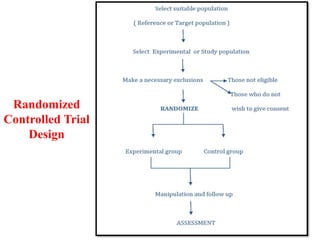
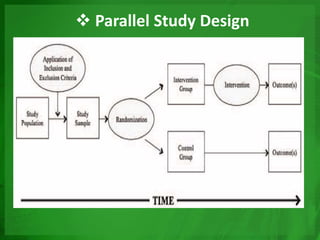
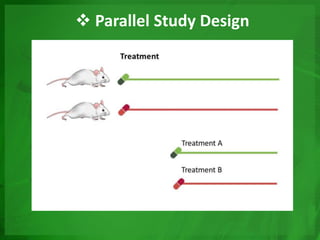
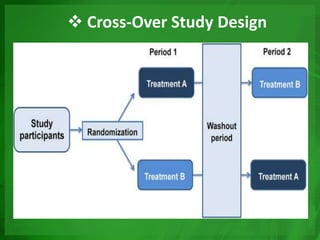
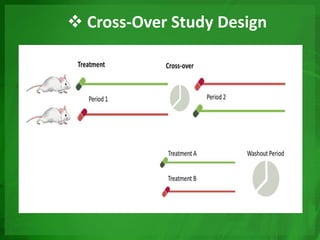
Experimental epidemiology involves controlled studies in which researchers introduce an intervention and observe its effects. Randomized controlled trials are considered the gold standard, as they randomly assign subjects to study and control groups to limit bias. This allows investigators to determine cause-and-effect relationships. Key features of randomized controlled trials include developing a study protocol, selecting and randomizing a population, implementing an intervention for the study group, following up with both groups, and assessing outcomes to compare results. Well-designed randomized controlled trials provide the strongest evidence for evaluating health interventions and establishing epidemiological relationships.