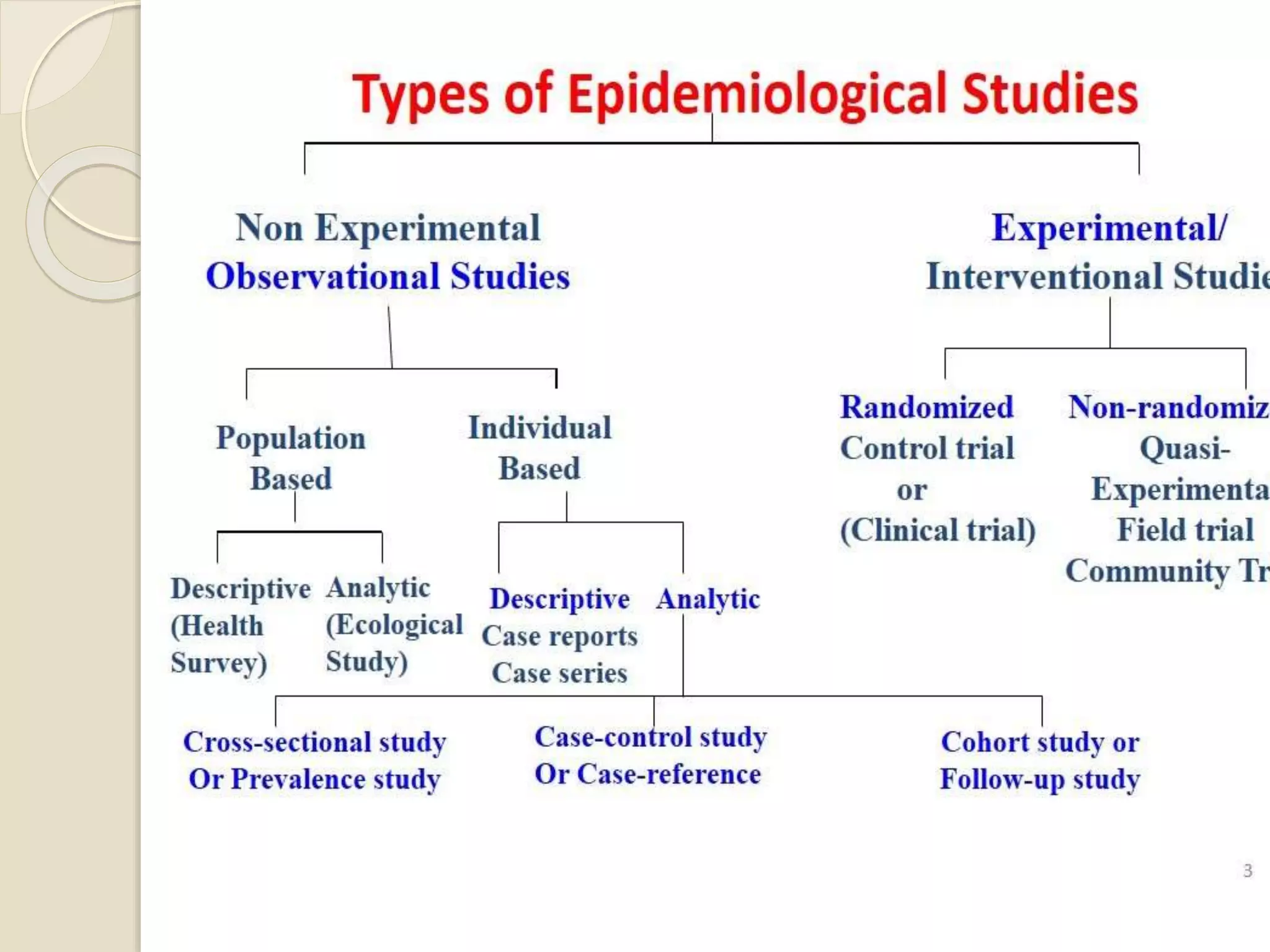
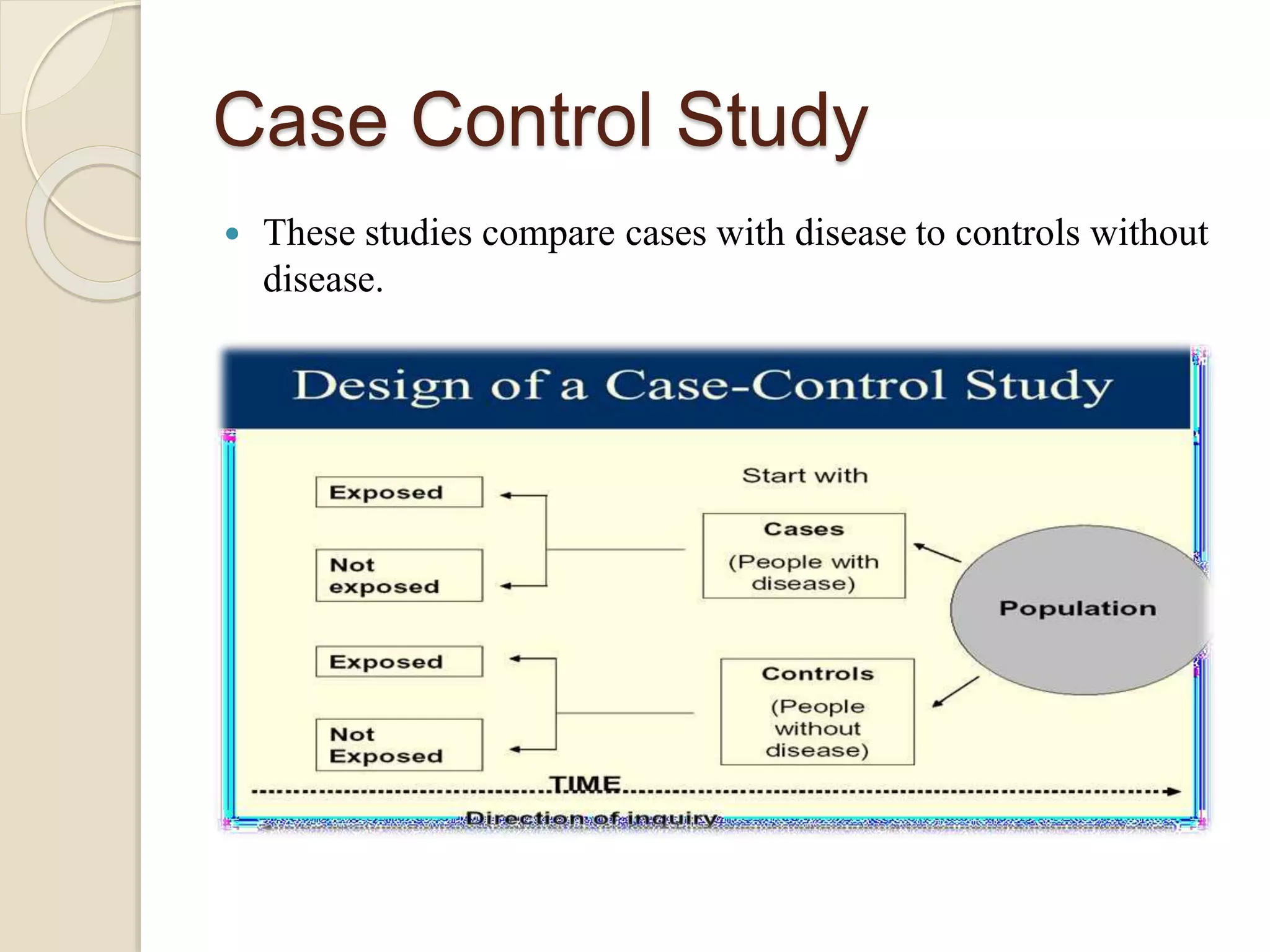
Pharmacoepidemiology is the study that applies epidemiological principles to pharmacology, focusing on the effects of market-approved drugs within populations. It includes various study types like observational and intervention studies, aiming to ensure drug safety, efficacy, and optimal use. The field benefits government agencies, healthcare practitioners, pharmaceutical companies, and consumers by providing crucial insights into drug utilization and safety.