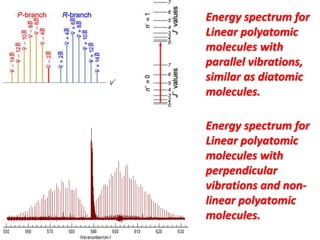
1) Vibrational spectra provides information about the molecular vibrations of a system by showing peaks that allow identification of components in a sample. Vibrational spectra can be seen in both diatomic and polyatomic molecules.
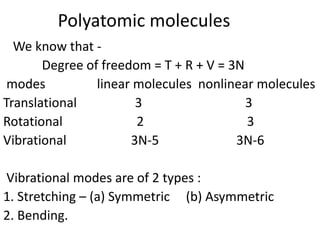
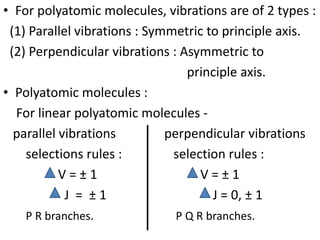
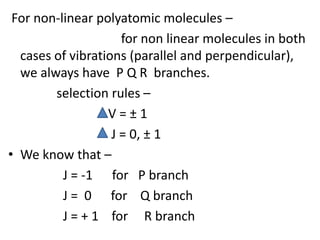
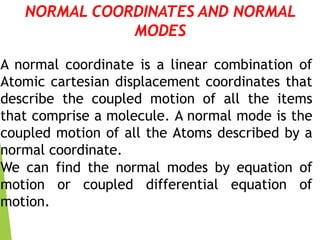
2) Polyatomic molecules have additional vibrational modes compared to diatomic molecules, including bond bending and asymmetric/symmetric stretching. Normal coordinates describe the coupled motion of atoms in a molecule as a linear combination of displacements.
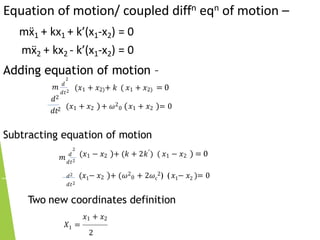
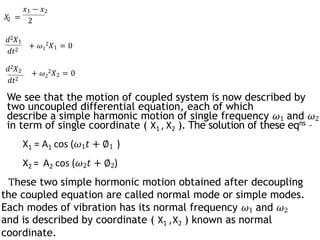
3) Solving the coupled differential equations of motion results in normal modes of vibration, which are uncoupled simple harmonic motions characterized by normal frequencies and normal coordinates. This decoupling describes the overall motion of the coupled molecular system.