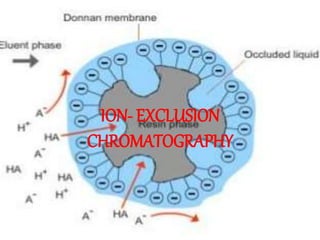
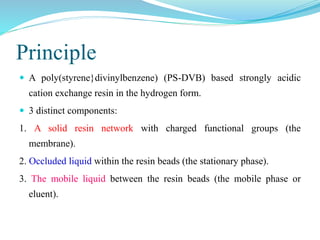
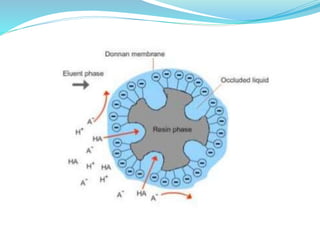
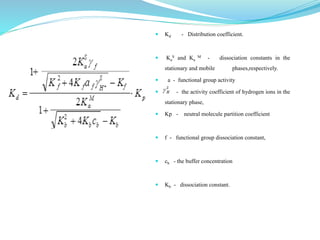
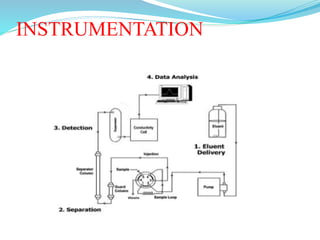
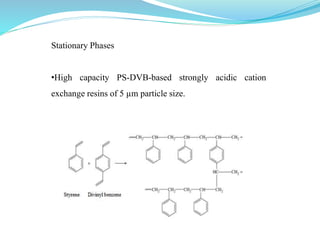
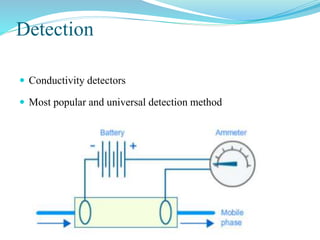
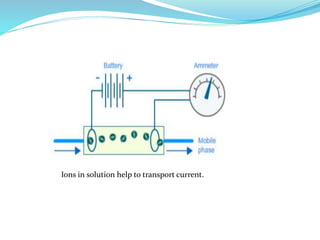
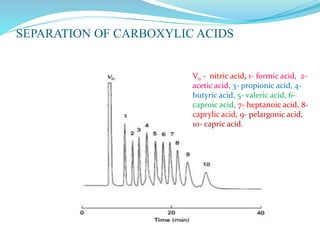
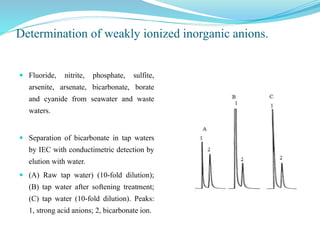
This document discusses ion exclusion chromatography, which uses an ion exchange stationary phase to separate ionic and nonionic substances. Ionic substances pass quickly through the column while nonionic substances are retained longer. Separation depends on whether substances are ionized and repelled by the resin or able to enter the resin network if nonpolar or partially ionized. Detection methods include conductivity detectors and UV-visible or fluorescence detectors. Applications include separation of carboxylic acids, inorganic anions, amino acids, and determination of water in organic solvents.