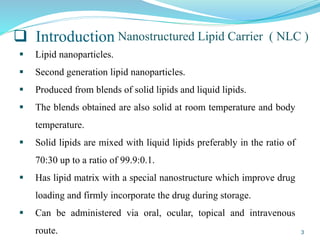
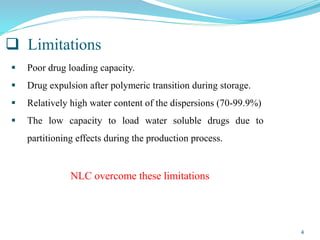
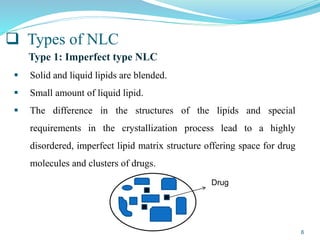
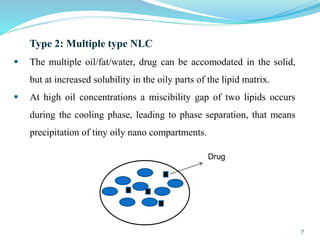
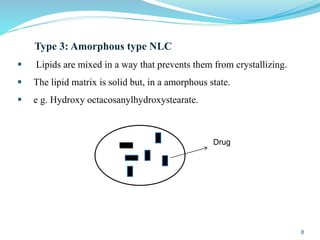
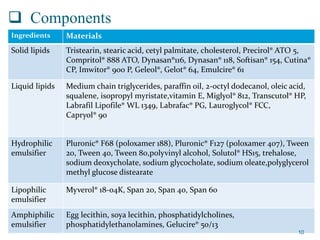
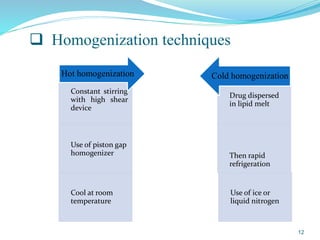
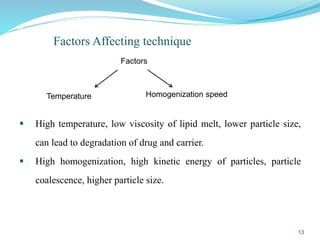
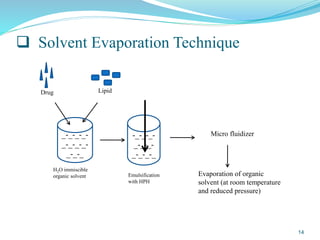
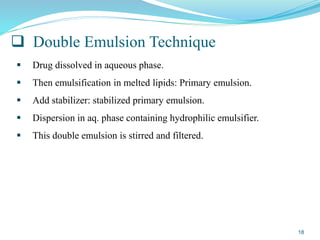
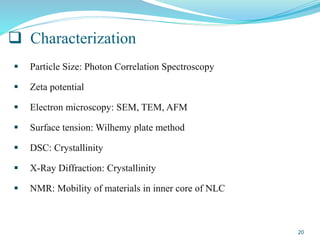
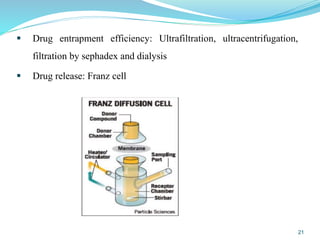
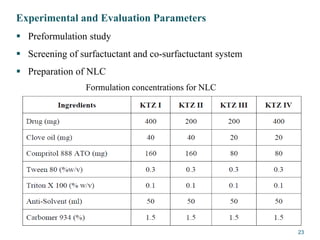
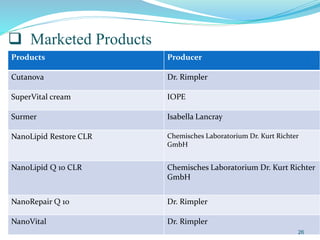
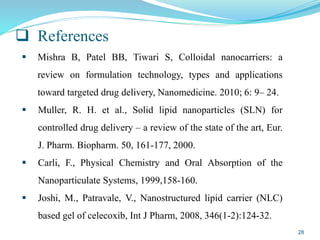
This document provides an overview of nanostructured lipid carriers (NLCs), including their advantages over other lipid nanoparticles, types of NLCs, composition, preparation methods, characterization techniques, marketed products, and conclusions. NLCs are produced from blends of solid and liquid lipids that form a solid matrix at body temperature with an imperfect structure allowing for high drug loading. They can be prepared using methods like homogenization, solvent evaporation, and melting dispersion. NLCs show potential for delivery of both lipophilic and hydrophilic drugs via various administration routes.