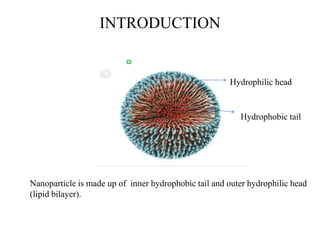
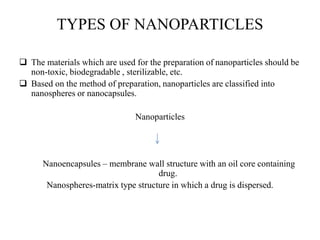
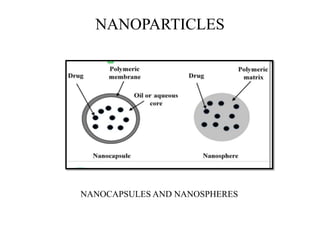
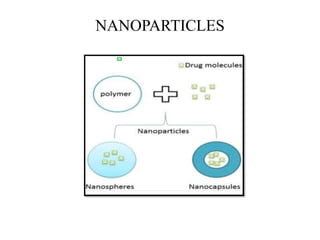
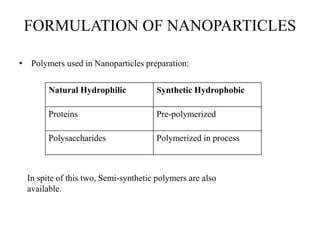
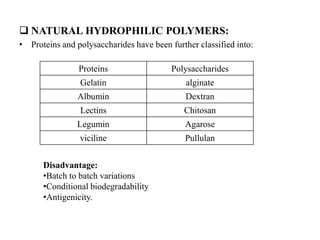
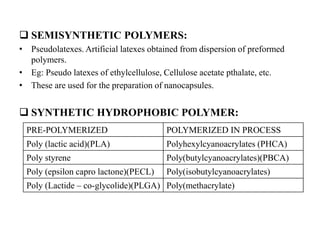
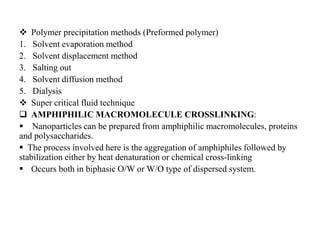
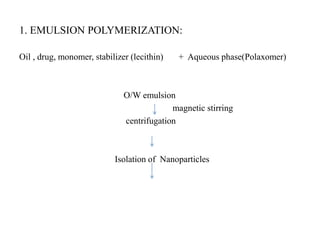
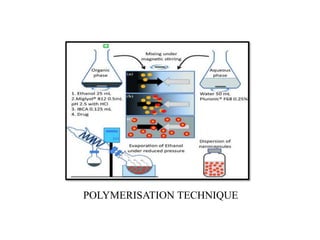
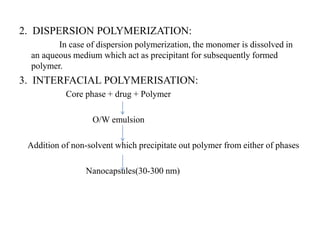
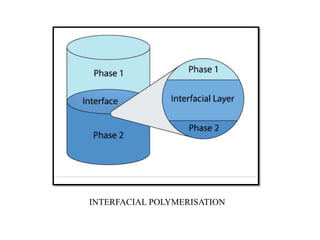
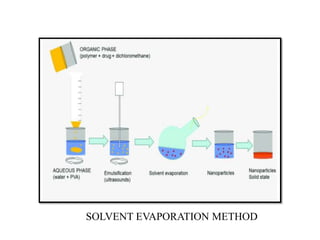
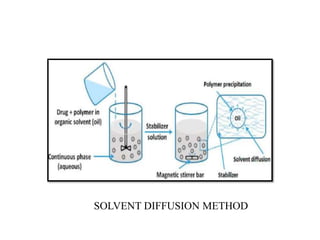
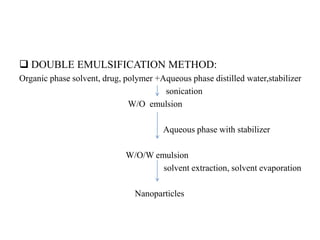
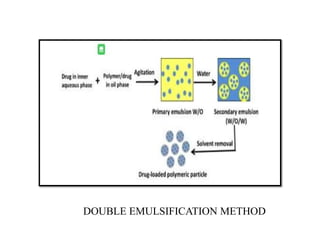
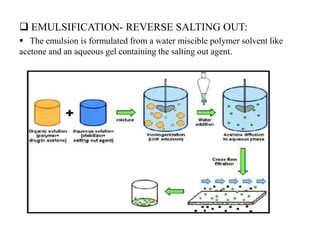
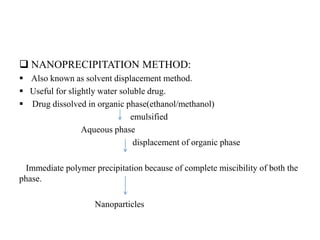
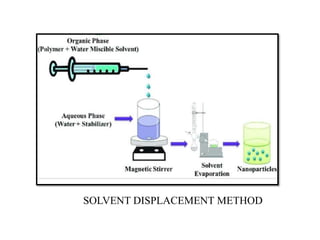
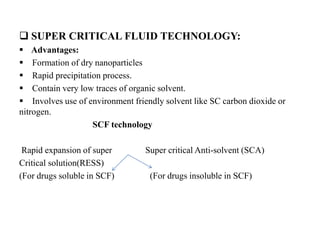
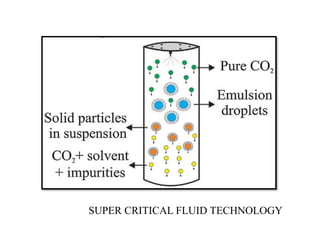
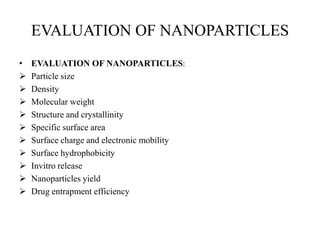
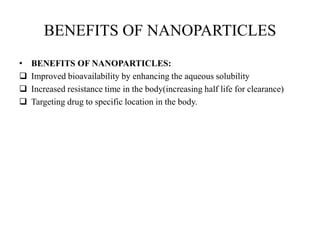
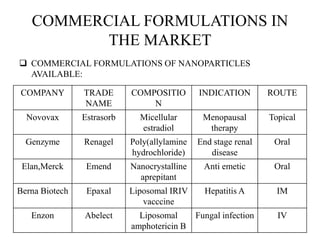
The document discusses nanoparticles and their use as drug delivery vehicles. It defines nanoparticles as solid colloidal particles between 1-1000 nm in size that can be used to encapsulate, dissolve or attach drugs. Nanoparticles are classified as nanospheres or nanocapsules depending on their structure and can be made from natural, synthetic or semi-synthetic polymers. The document discusses various methods for formulating nanoparticles, including polymerization, solvent evaporation and supercritical fluid technology. The advantages of nanoparticles for drug delivery are also summarized.