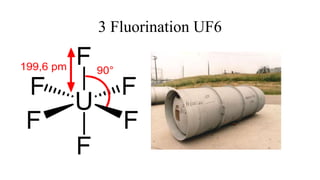
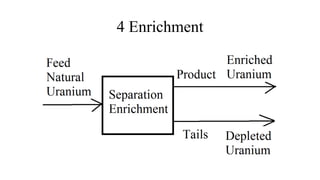
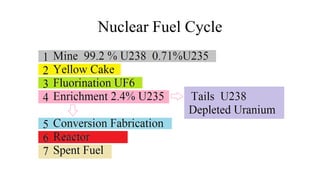
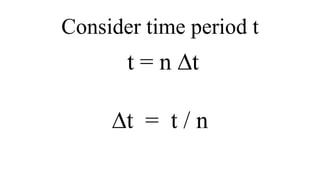This document provides an overview of nuclear chemistry concepts including:
- Subatomic particles like protons, neutrons, and electrons
- Isotopes and nuclides which have the same number of protons but different neutrons
- Radioactive decay processes like alpha, beta, and gamma emissions
- Nuclear reactions like fusion and fission that change one element into another and release energy
- Applications of nuclear chemistry concepts like carbon dating and the nuclear fuel cycle for power generation