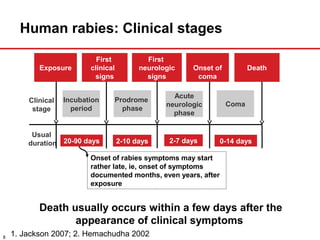
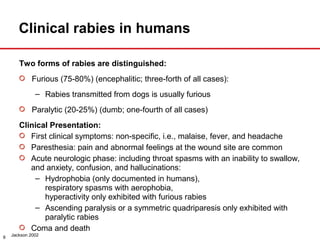
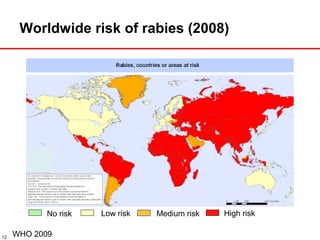
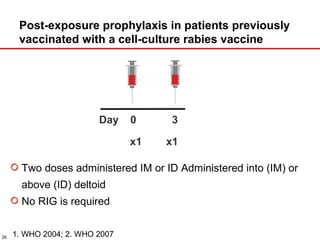
The document presents a detailed overview of rabies, including its pathogenesis, clinical stages, and prevention strategies. It highlights the global epidemiology of rabies, particularly in India, and discusses various rabies vaccines and post-exposure prophylaxis guidelines. Additionally, it emphasizes the importance of immediate wound treatment and the administration of rabies vaccines to prevent this virtually fatal disease.