This document discusses rabies, a viral disease transmitted through animal bites that affects the central nervous system. Some key points:
- Rabies causes 59,000 human deaths annually, mostly in Africa and Asia. Dogs are responsible for 99% of human rabies through bites.
- After an incubation period, symptoms include hyperactivity, hydrophobia, and paralysis. It is almost always fatal without post-exposure prophylaxis.
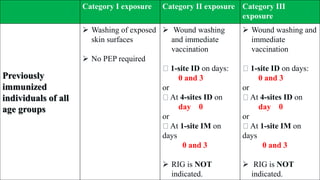
- Louis Pasteur developed the first rabies vaccine in 1890. Modern cell-culture vaccines have replaced nerve tissue vaccines. Post-exposure prophylaxis includes wound cleaning and a series of vaccine doses, with rabies immunoglobulin for severe exposures.
- In India, rabies causes