Embed presentation

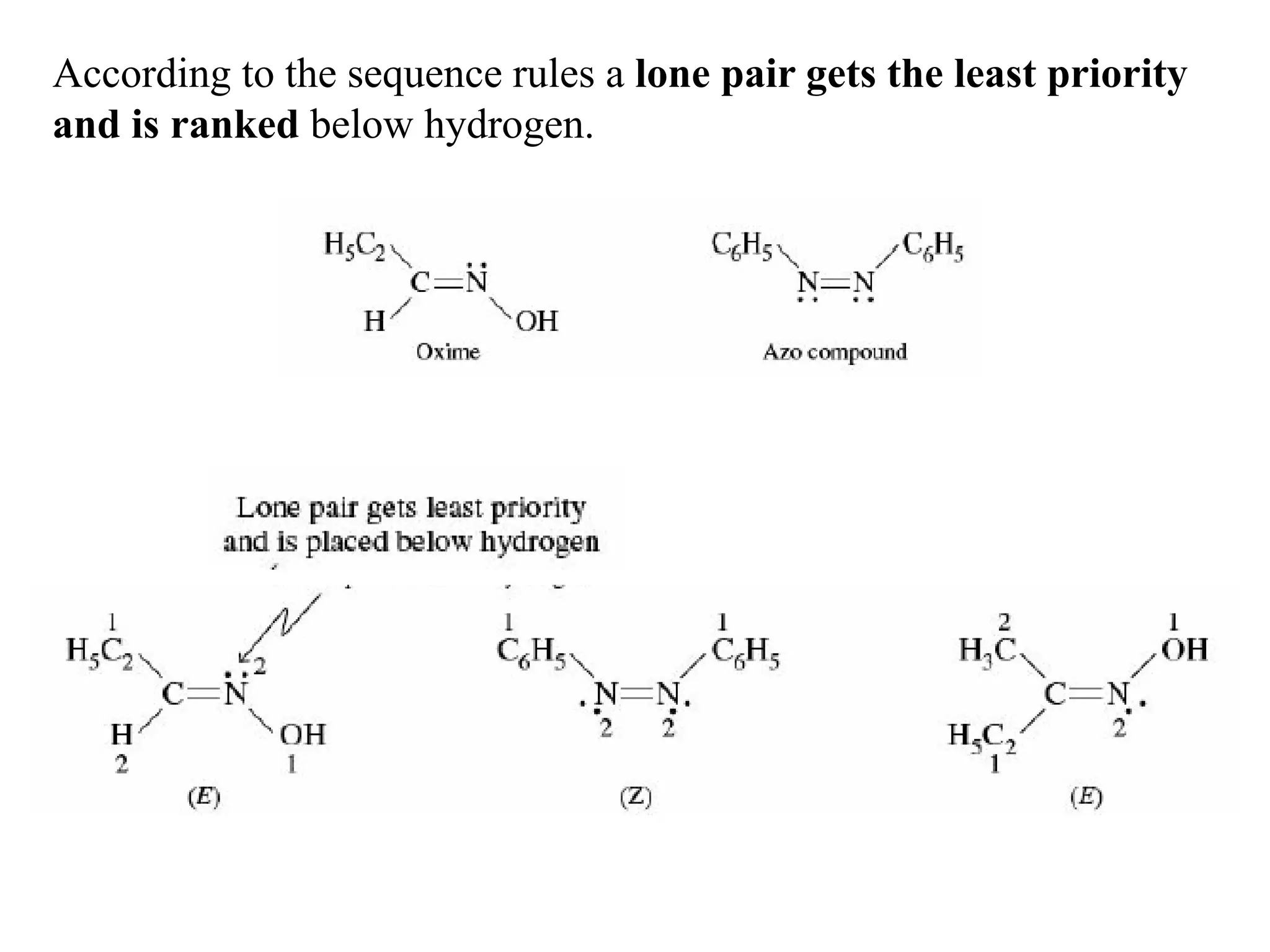
![Geometrical isomerism is not restricted to carbon–carbon double
bond [C=C ] but is also exhibited by compounds having a carbon–
nitrogen double bond [C=N–] as in oximes, or nitrogen–nitrogen
double bond [–N=N–] as in azo.](https://image.slidesharecdn.com/geometricalisomerism-200409062051/75/Geometrical-isomerism-7-2048.jpg)

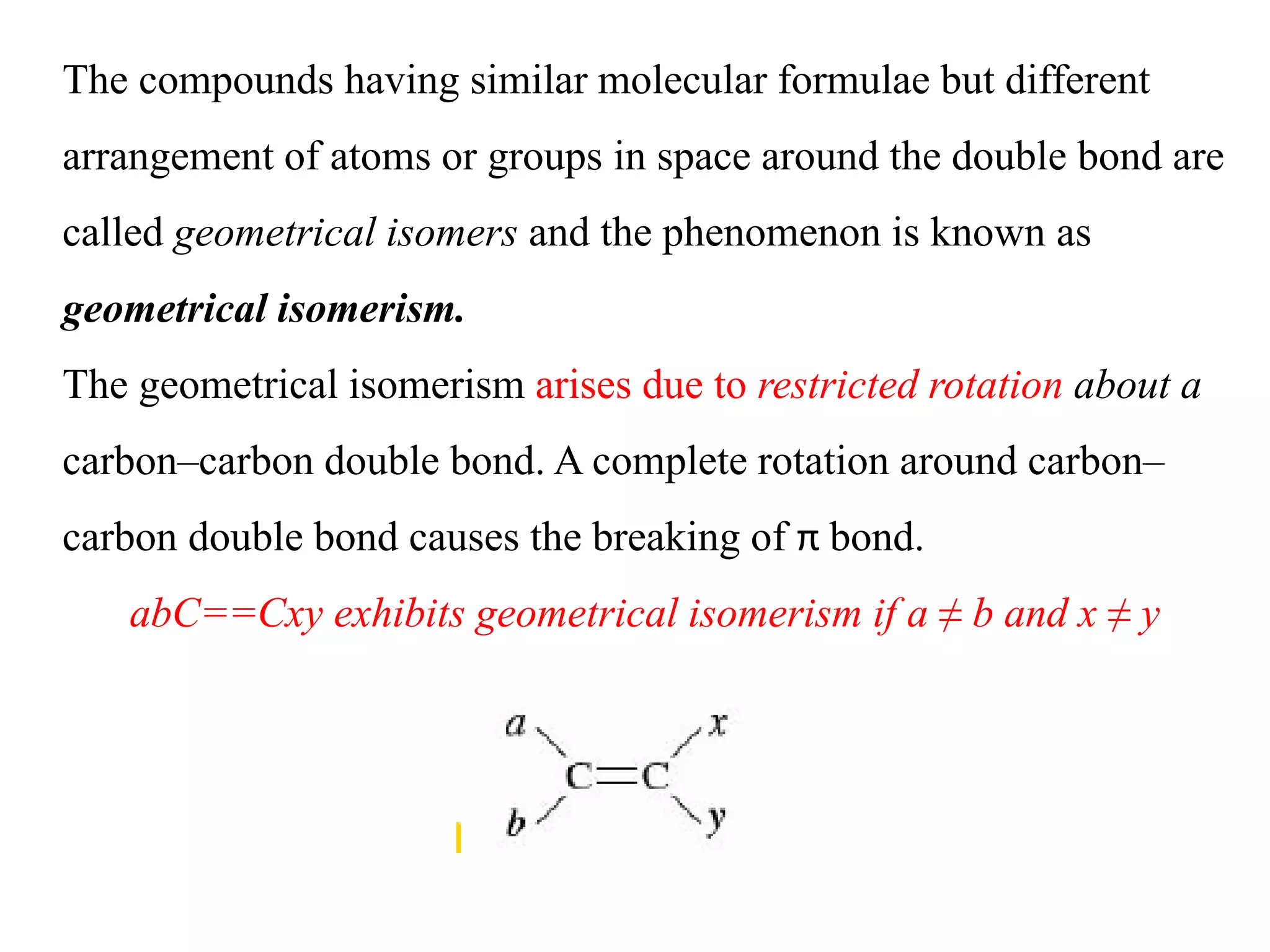
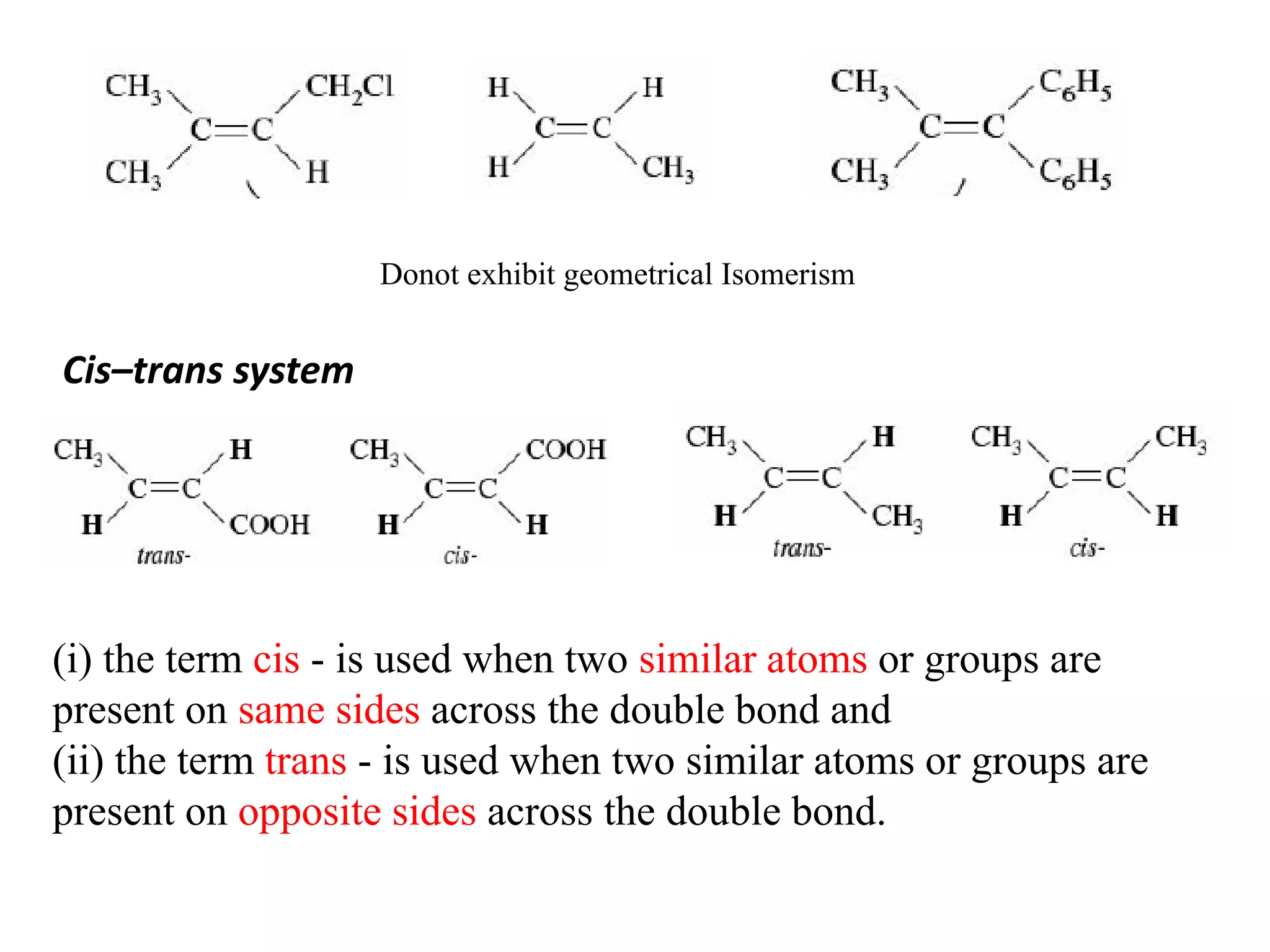
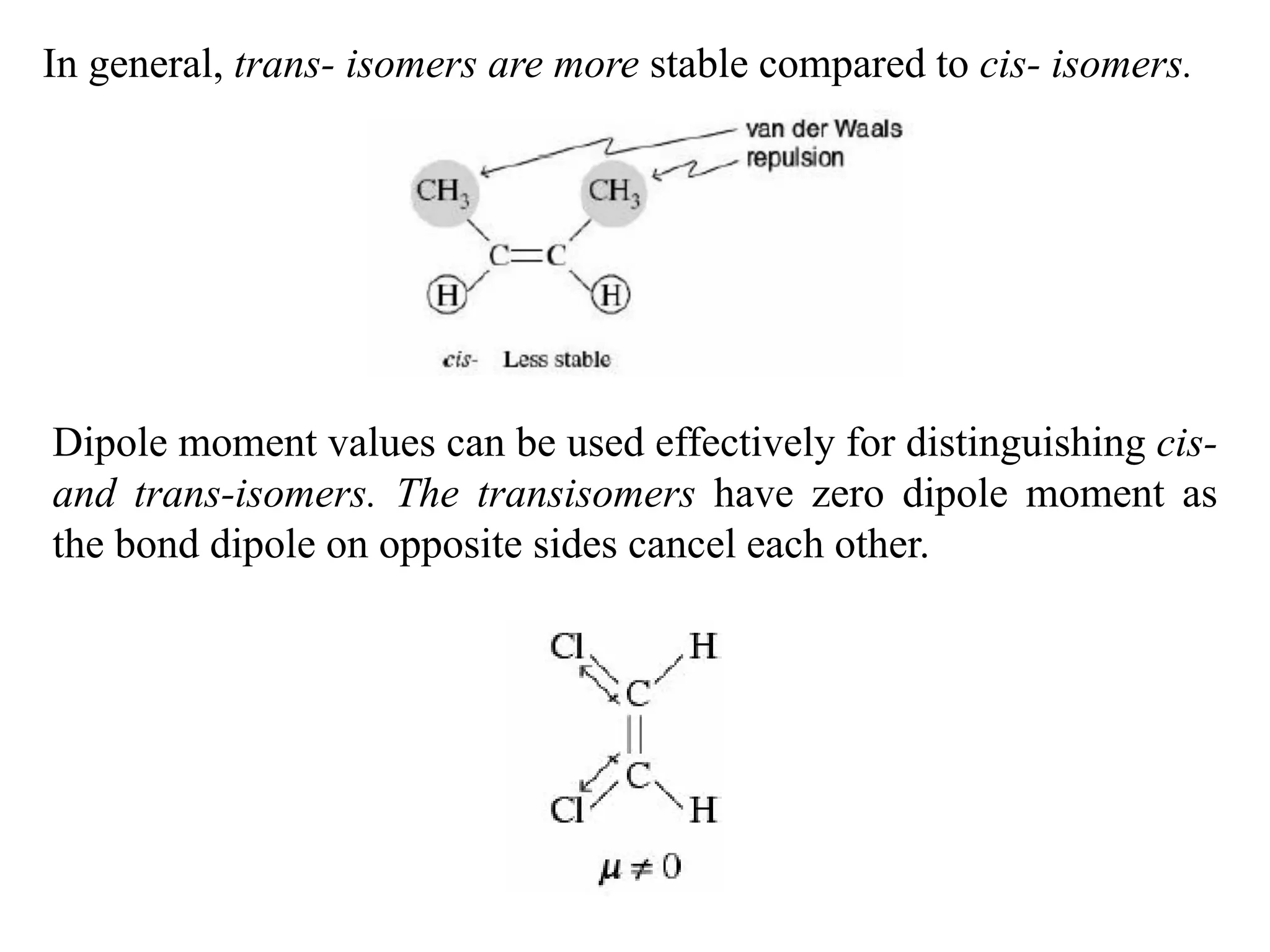
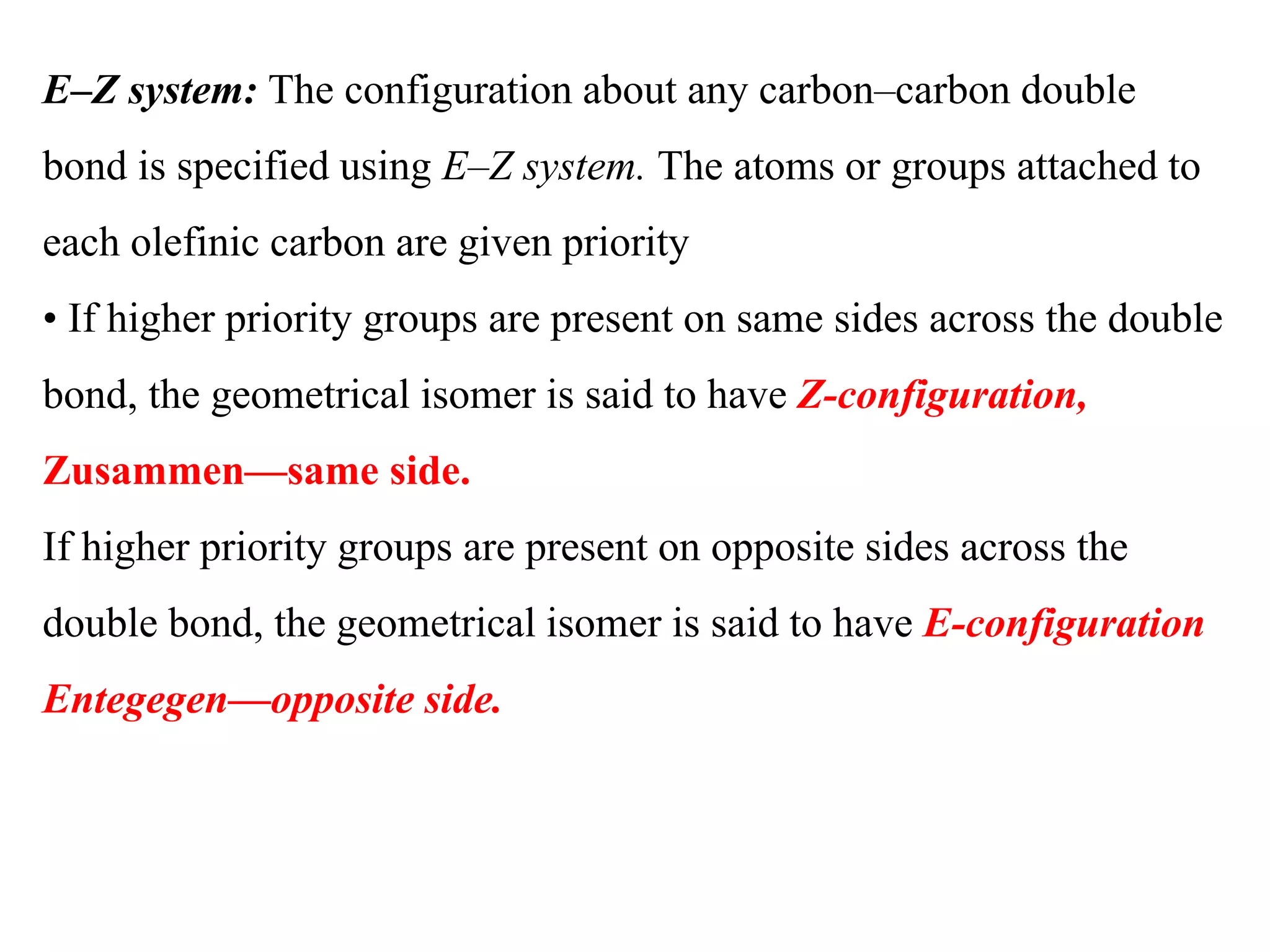
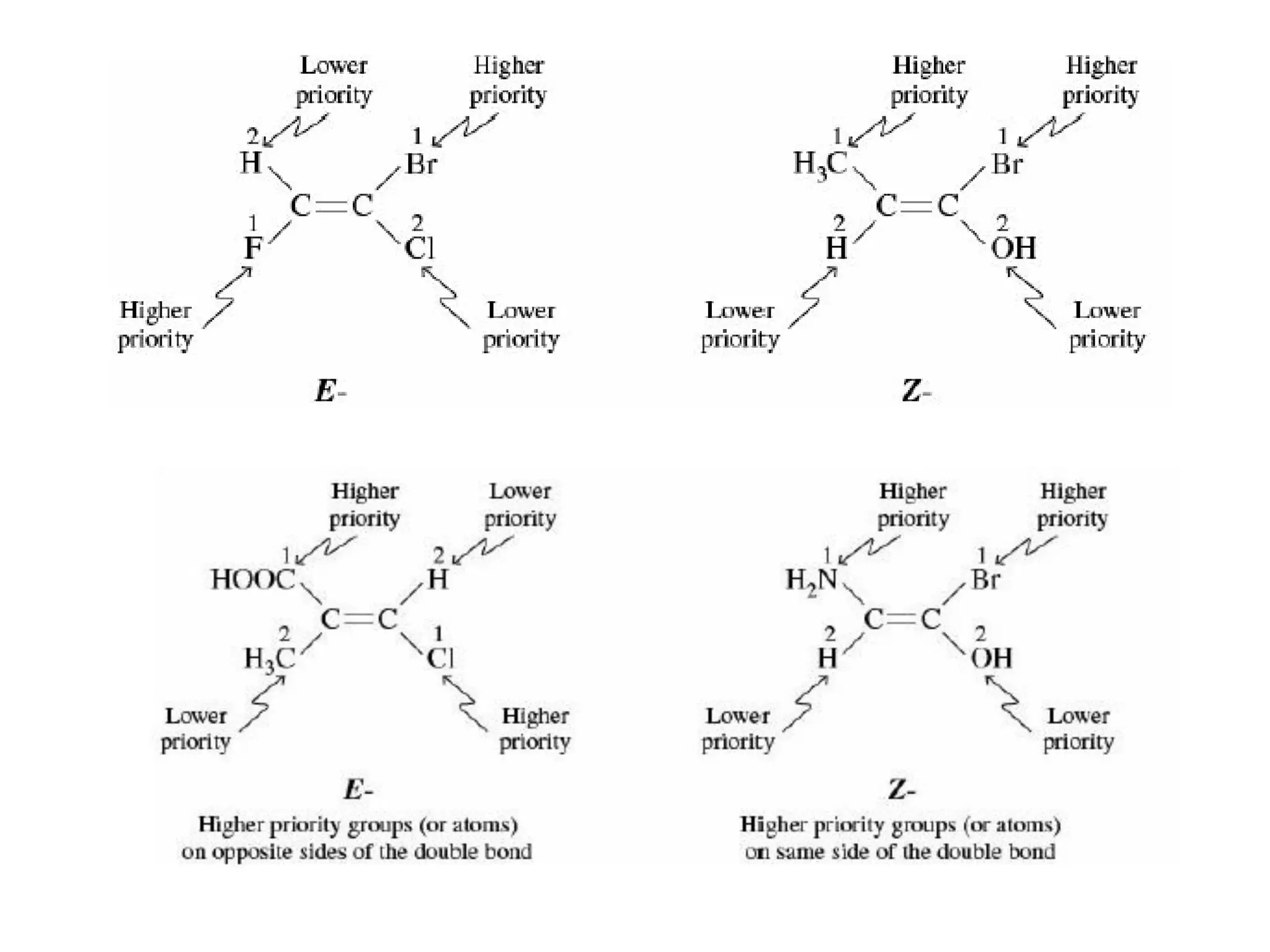
Geometrical isomers are compounds with the same molecular formula but different spatial arrangements of atoms or groups around a double bond. Geometrical isomerism arises from the inability of groups on opposite sides of a carbon-carbon double bond to rotate freely. Systems exhibiting geometrical isomerism include cis-trans isomers, where similar groups are on the same (cis) or opposite (trans) sides of the double bond, and E-Z isomers, where priority rules determine if higher priority groups are on the same (Z) or opposite (E) sides. Geometrical isomerism also occurs in compounds containing carbon-nitrogen or nitrogen-nitrogen double bonds.






![Geometrical isomerism is not restricted to carbon–carbon double
bond [C=C ] but is also exhibited by compounds having a carbon–
nitrogen double bond [C=N–] as in oximes, or nitrogen–nitrogen
double bond [–N=N–] as in azo.](https://image.slidesharecdn.com/geometricalisomerism-200409062051/75/Geometrical-isomerism-7-2048.jpg)
