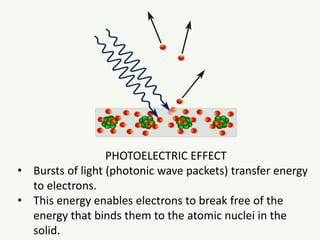
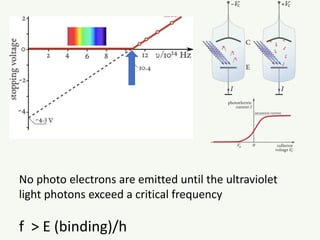
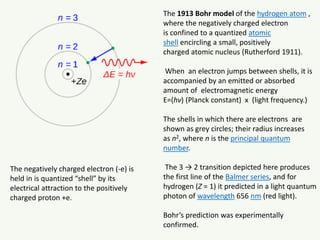
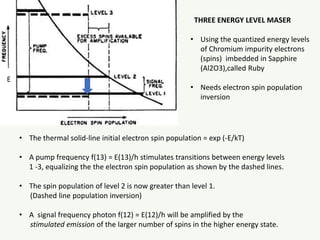
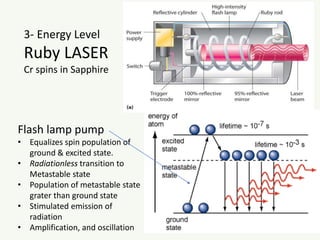
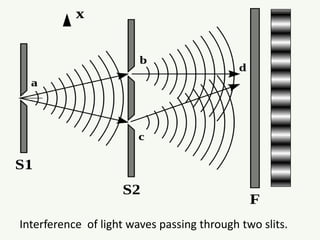
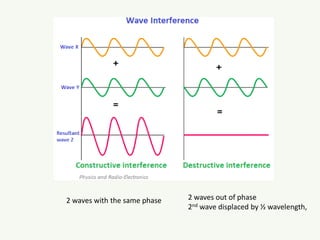
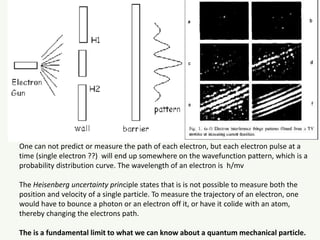
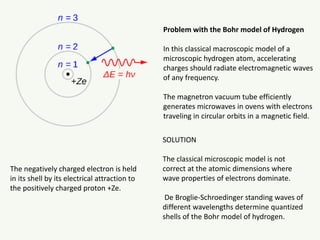
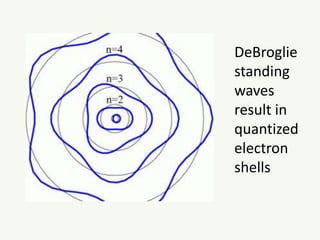
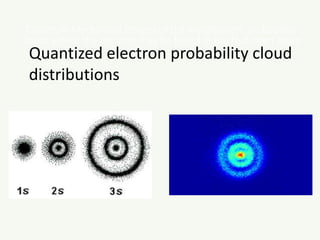
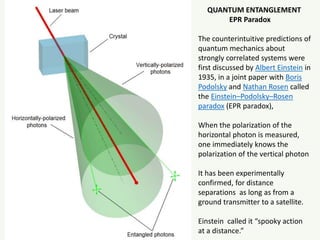
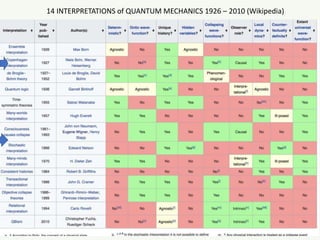
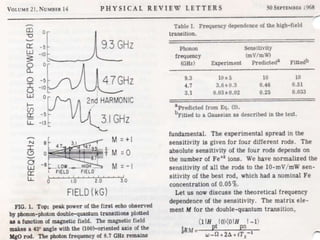
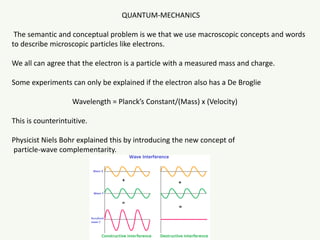
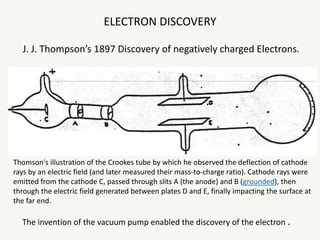
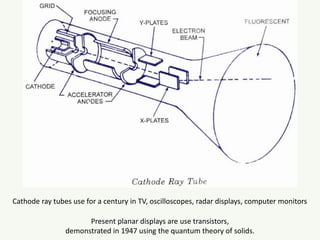
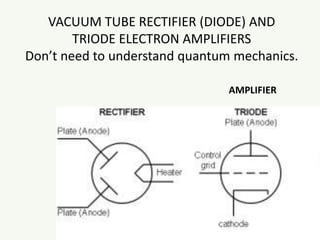
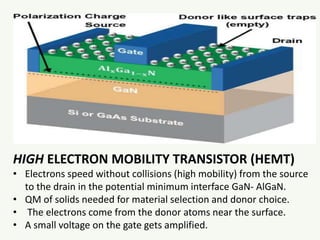
The document discusses the foundational developments in quantum mechanics, particularly focusing on the discovery and properties of electrons, the invention of transistors, and the creation of lasers. It highlights significant milestones in quantum theory, interpretations of quantum mechanics, and the dual wave-particle nature of electrons, emphasizing the historical contributions of notable physicists like J.J. Thomson, Albert Einstein, and Niels Bohr. Additionally, it touches on contemporary interpretations of quantum mechanics and theories connecting consciousness with quantum events.




![Drawing of a photon (in green) being emitted from carbon molecules. [Image
source: Nancy Ambrosiano, Los Alamos National Laboratory, July 2017 News
Release, “Single-photon emitter has promise for quantum info-processing,” (Public
domain)] PHOTON PULSE OF PARTICLE-LIKE LIGHT](https://image.slidesharecdn.com/aspecqmelectronics-210223201023/85/Quantum-Mechanics-Electrons-Transistors-LASERS-10-320.jpg)