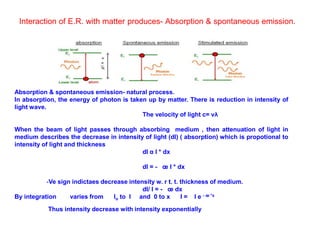
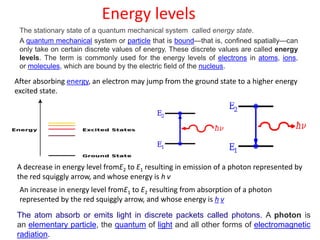
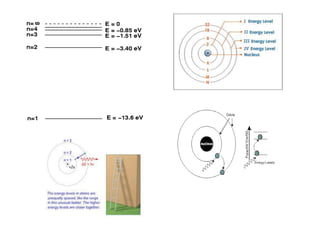
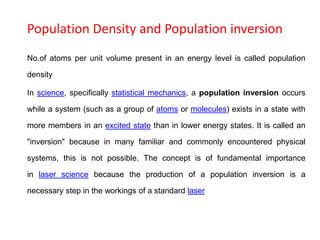
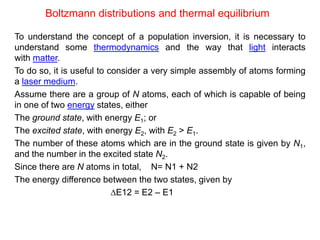
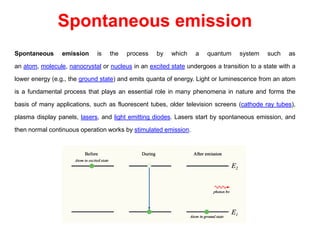
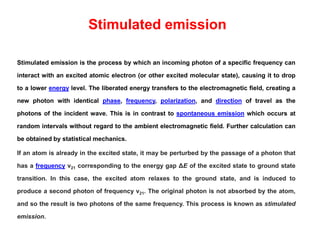
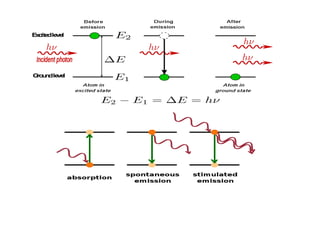
The document provides a comprehensive overview of lasers, detailing their historical development, fundamental principles of operation, and key characteristics compared to ordinary light. It describes the process of stimulated emission, which is essential for laser functionality, and explores the interaction of electromagnetic radiation with matter. Additionally, the document discusses concepts like population inversion, absorption, and spontaneous emission, significant for understanding laser technology.












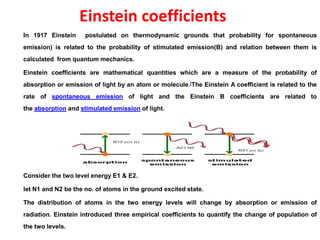

![At thermodynamic equilibrium, the net change in the number of any excited atoms is
zero, being balancing, by loss and gain due to all process
0 = - B12 ρ(ν) N1 + B21 ρ(ν) N2 + A21 N2
B12 ρ(ν) N1 - B21 ρ(ν N2 = A21 N2
ρ(ν) = A21 N2 / ( B12 N1 - B21 N2)
= (A21/B12) / [ ( N1/N2) –(B21/B12)]
= (A21/B12) / [ ( exp((E2-E1/KT)) –(B21/B12)]
According to Plank’s radiation law for any value of T
A21/B12 = (8πһν3μ3)/c3
ρ(ν) = (8πһν3μ3)/c3 / [ ( exp((E2-E1/KT)) –(B21/B12)]
When an atom with two energy levels is placed in the radiation field then
B12= B21
ρ(ν) = (8πһν3μ3)/c3 / [ ( exp((E2-E1/KT)) –1]
B12, B21 and A12 are known as Einstein coefficient.](https://image.slidesharecdn.com/presentation-laser-160917130701/85/Presentation-laser-25-320.jpg)
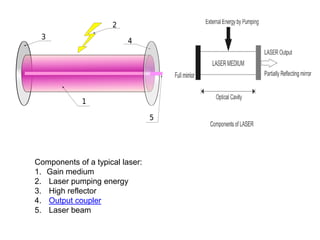
![A laser consists of a gain medium, a mechanism to energize it, and something to
provide optical feedback.[7] The gain medium is a material with properties that allow it
to amplify light by way of stimulated emission. Light of a specific wavelength that
passes through the gain medium is amplified (increases in power).
For the gain medium to amplify light, it needs to be supplied with energy in a process
called pumping. The energy is typically supplied as an electric current or as light at a
different wavelength. Pump light may be provided by a flash lamp or by another laser.
The most common type of laser uses feedback from an optical cavity—a pair of
mirrors on either end of the gain medium. Light bounces back and forth between the
mirrors, passing through the gain medium and being amplified each time. Typically one
of the two mirrors, theoutput coupler, is partially transparent. Some of the light escapes
through this mirror. Depending on the design of the cavity (whether the mirrors are flat
or curved), the light coming out of the laser may spread out or form a narrow beam. In
analogy to electronic oscillators, this device is sometimes called a laser oscillator.
Most practical lasers contain additional elements that affect properties of the emitted
light, such as the polarization, wavelength, and shape of the beam.](https://image.slidesharecdn.com/presentation-laser-160917130701/85/Presentation-laser-26-320.jpg)