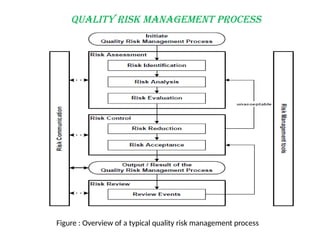
Quality risk management (QRM) is a systematic approach to identify, assess, control, and communicate risks throughout the drug product lifecycle to maintain product quality. It is based on scientific knowledge, aiming to protect patient safety, and involves a process of risk assessment, risk control, risk communication, and risk review. Effective QRM supports better decision-making in the industry and regulatory environments, ensuring consistent quality and compliance.