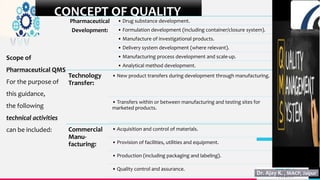
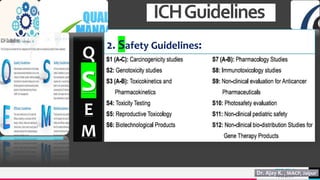
The document discusses the significance of Quality Management Systems (QMS) in the pharmaceutical industry, focusing on guidelines from the US FDA and the International Council for Harmonization (ICH). It emphasizes the importance of maintaining product quality, safety, and efficacy amidst globalization and market competition, while outlining key concepts such as quality assurance, quality control, and the factors contributing to quality variation. Additionally, it covers the scope of QMS in drug development, manufacturing, and the adherence to established quality, safety, and efficacy guidelines.