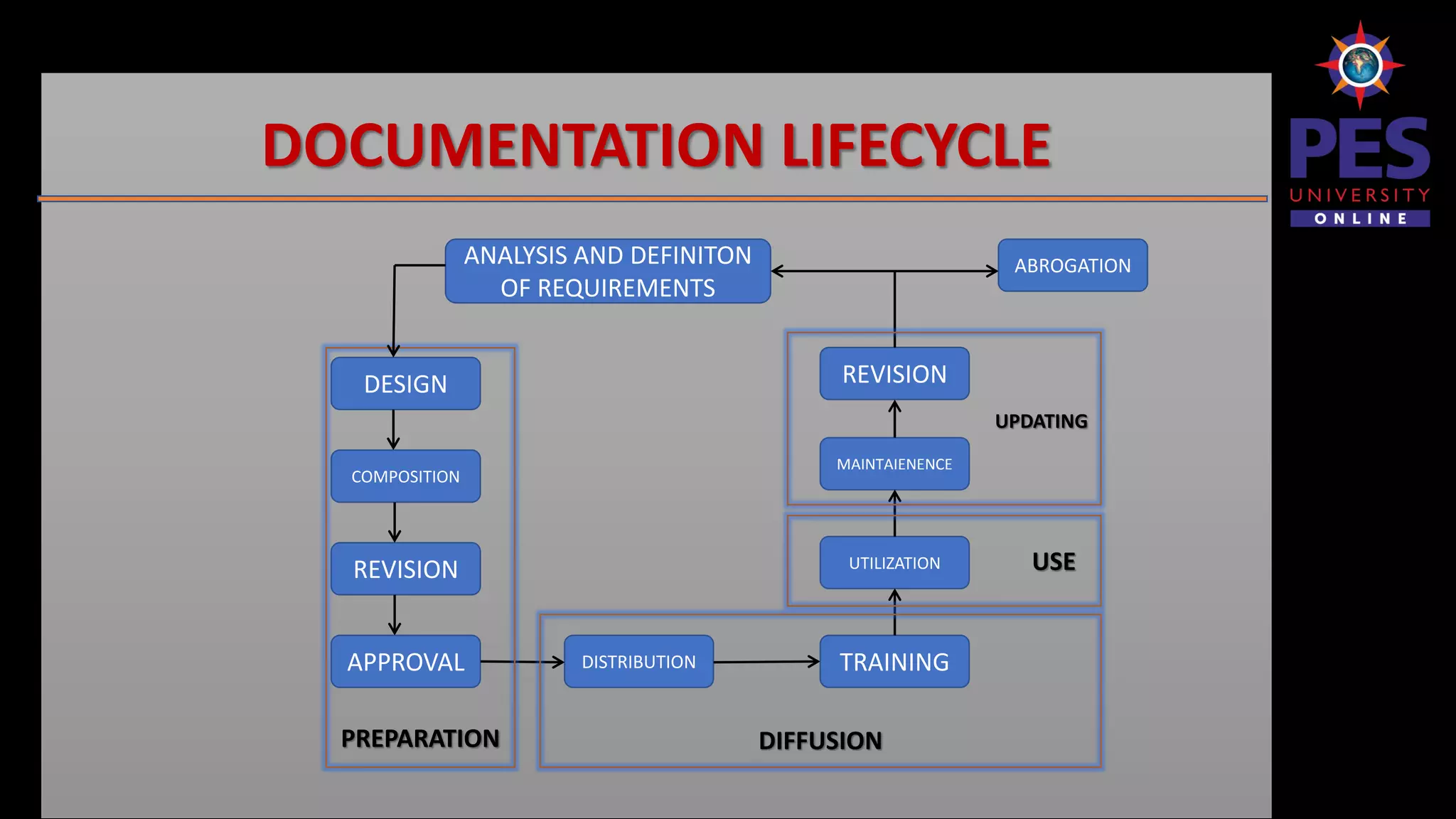
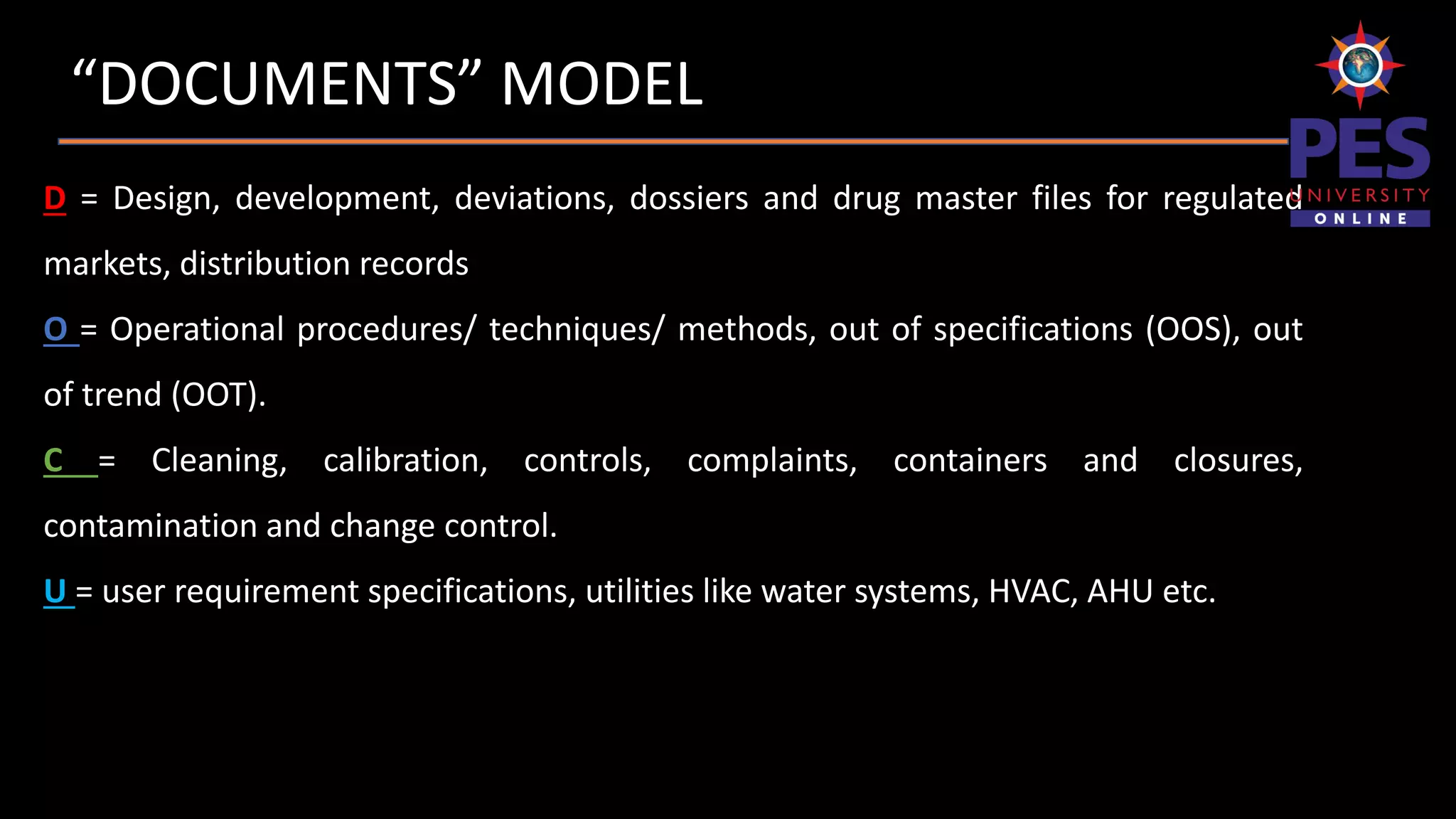
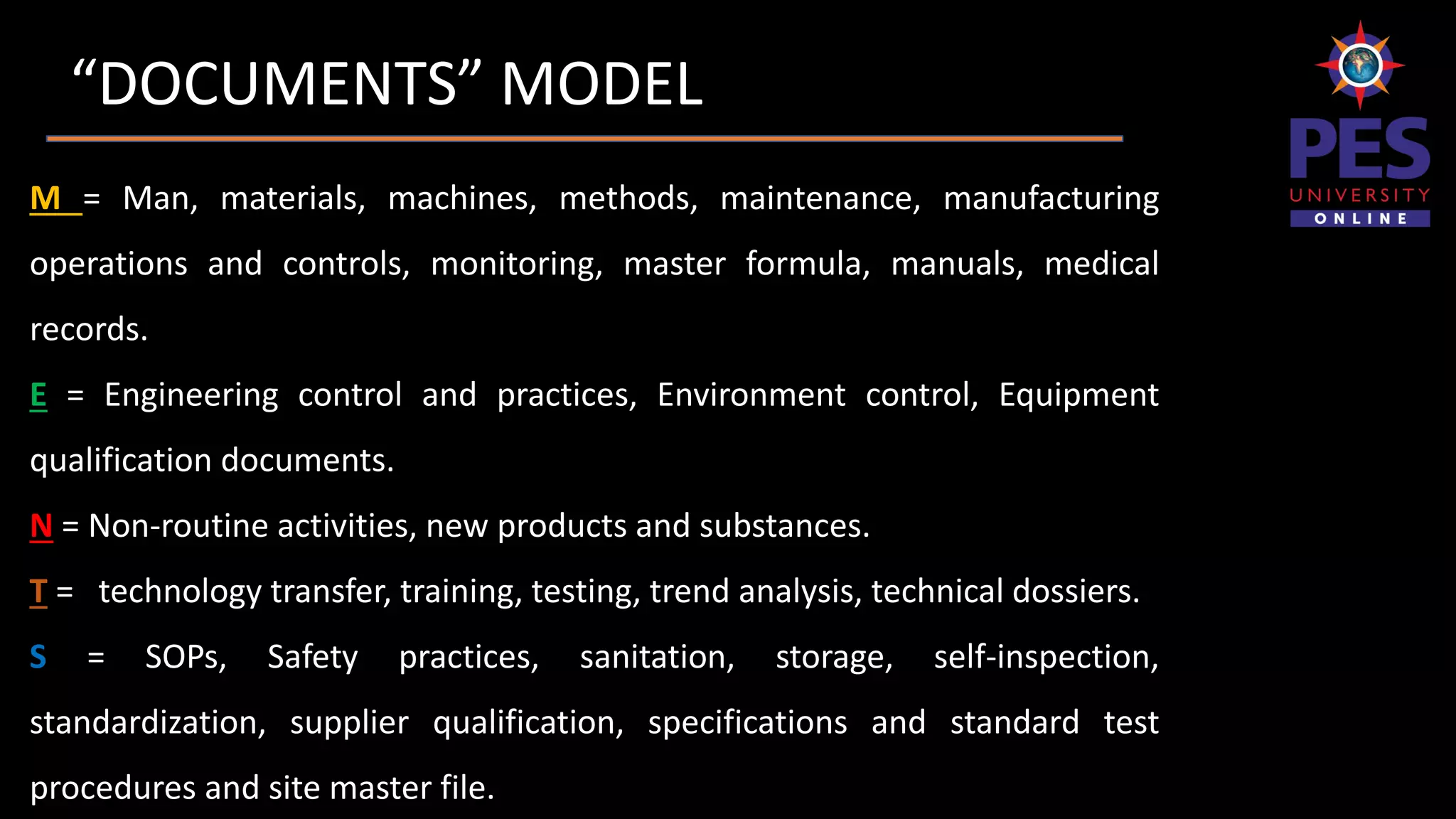
The document outlines the importance and structure of documentation in the pharmaceutical industry, emphasizing its necessity for compliance with regulatory guidelines, maintaining quality control, and ensuring traceability of processes. It details various types of documents, including quality manuals, standard operating procedures (SOPs), and master formula records, alongside the characteristics and lifecycle of effective documentation. Additionally, the document discusses the auditing process, specification criteria, and distribution record requirements to facilitate efficient quality management systems.