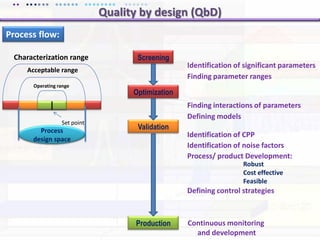
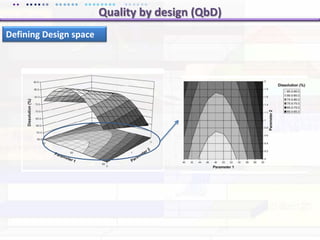
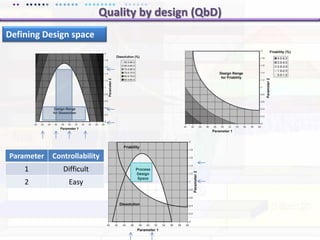
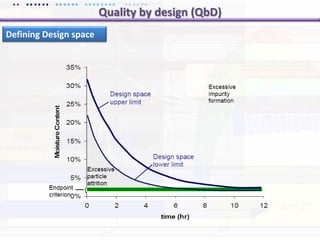
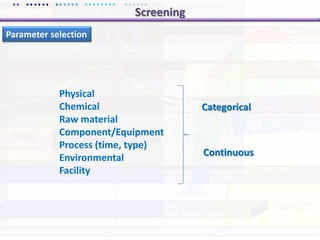
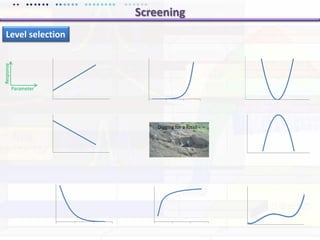
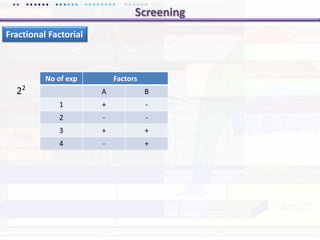
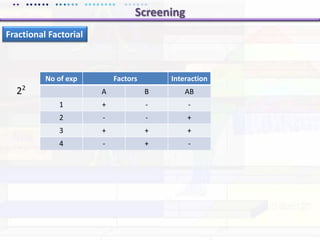
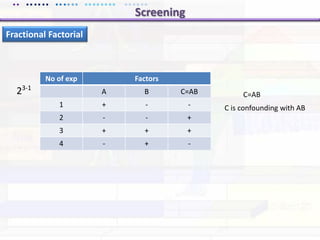
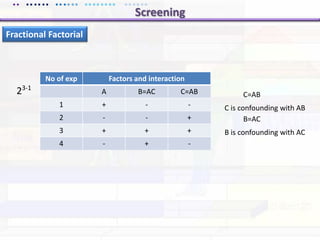
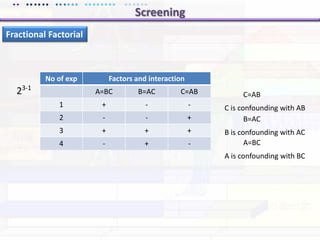
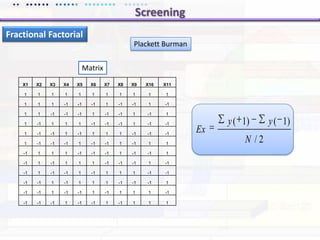
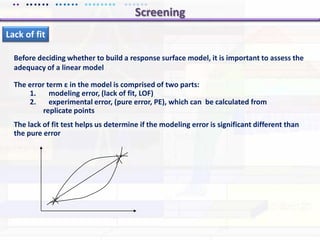
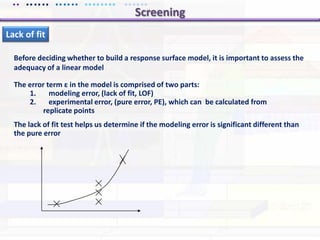
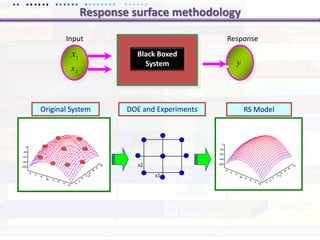
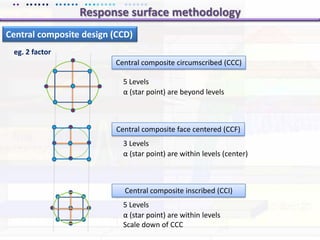
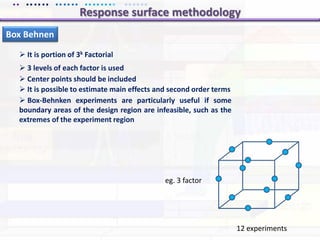
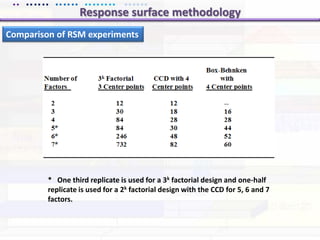
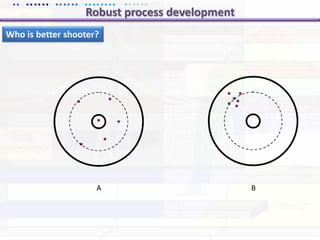
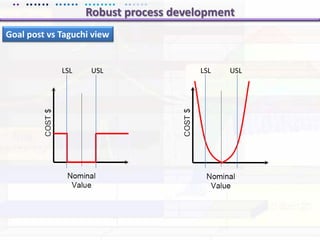
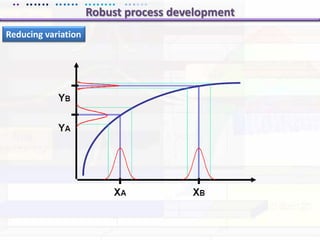
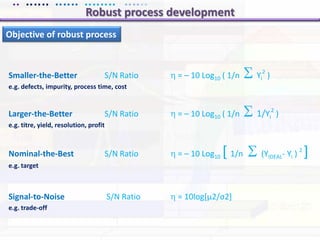
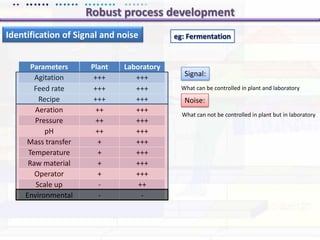
This document discusses quality by design (QbD) approaches for biopharmaceutical development. QbD focuses on designing quality into the product and process based on an understanding of critical quality attributes and critical process parameters. Key aspects of QbD include identifying critical attributes and parameters, using tools like design of experiments to understand their impact, defining a design space, and ensuring robustness through continuous monitoring and improvement. Statistical tools and multidisciplinary teams are important for successful QbD implementation.