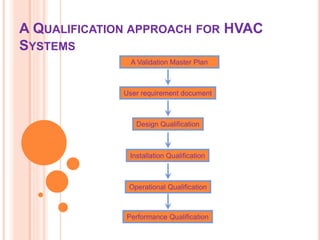
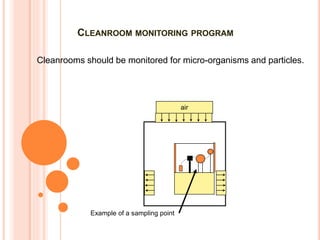
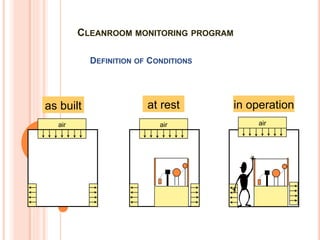
The document details the qualification, maintenance, and inspection processes for HVAC systems, particularly in pharmaceutical environments. It outlines key definitions and qualifications including Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ), emphasizing the systematic approach necessary for compliance with Good Manufacturing Practices (GMP). The importance of validation, documentation, and risk management in maintaining efficient HVAC systems is reiterated throughout.