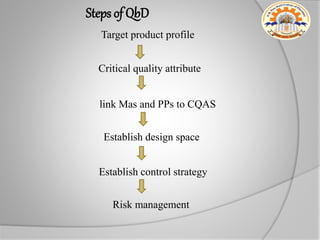
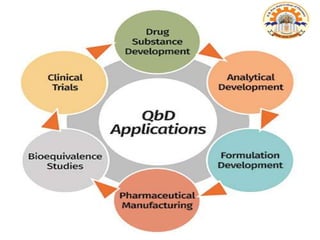
The document discusses the concept of Pharmaceutical Quality by Design (QbD), which is a systematic approach to pharmaceutical development that emphasizes product and process understanding based on sound science and quality risk management. QbD aims to design quality products and manufacturing processes that consistently deliver intended performance, following ICH guidelines Q8 on pharmaceutical development, Q9 on quality risk management, and Q10 on pharmaceutical quality systems. Implementing QbD provides benefits like eliminating batch failures, minimizing deviations, and ensuring consistent product quality.