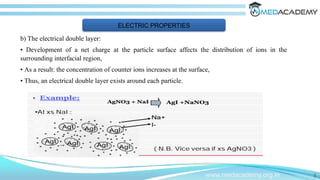
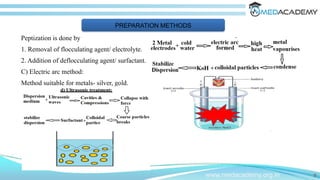
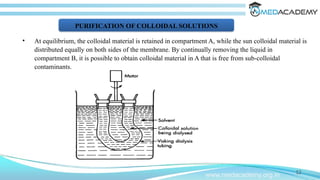
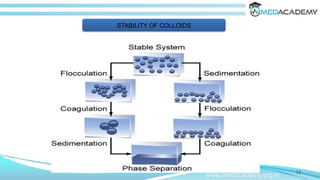
The document discusses the electrical properties of interfaces in colloidal systems, focusing on the mechanisms of surface charge acquisition such as ion dissolution, ionization, and ion adsorption. It also explains the concept of the electrical double layer and describes various methods for preparing and purifying colloidal solutions, including dialysis, electrodialysis, and ultrafiltration. Additionally, the document highlights the stability of colloids and their various pharmaceutical applications, emphasizing their roles in enhancing the effectiveness of certain compounds.