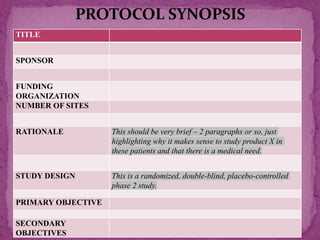
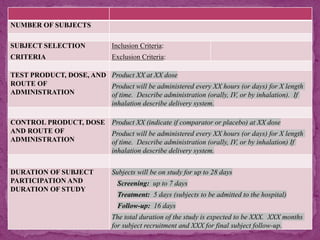
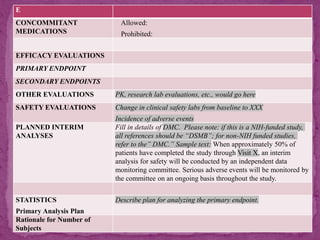
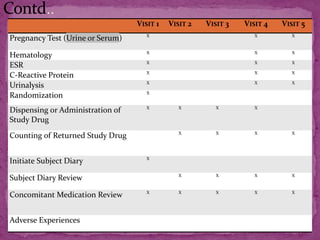
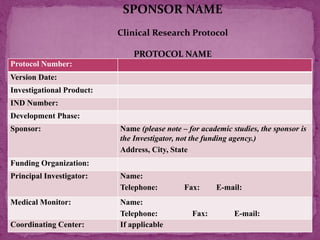
This document outlines a clinical research protocol template. It begins with an introduction section defining clinical research and clinical trials. It then describes the purpose and contents of a clinical research protocol, including sections for objectives, background/rationale, study design, eligibility criteria, treatments, assessments, data collection and analysis, monitoring, ethics and regulations. The protocol template provides guidance on the level of detail needed for each section to clearly explain the research question, methodology, and procedures to ensure scientific validity and participant safety.














































![leifu b|Jod'k:yftf /f]uL kfbrt'i6od . u'0fTjsf/0f+ 1]o+ ljsf/ Jo'kzfGto] .
r=;"=(.@
>'t] ko{jbftTj+ ax'zf] b[i6sd{tf . bfIo+ zf}rldlt 1]o+ j}B]u'0frt'i6od .
r=;"= (.^
pkrf/1tf bfIodg'/fuZr et{l/ . zf}r+ r]lt rt'isf]˜o+ u'0fM kl/r/]hg] ..
r=;"= (.*
ax'tf tq of]UoTjdg]sljwsNkgf . ;DkRr]lt rt'isf]˜o+ b|Jof0ff+u'0f pRot] ..
r=;"=(.&
:d[ltlgb]{zsfl/TjdeL?Tjdyflk r . 1fksTj+ r /f]uf0ffdft'/:o u'0ffM :d[tfM ..
r=;"= (.(](https://image.slidesharecdn.com/clinicalresearchprotocol-160623145316/85/Clinical-research-protocol-54-320.jpg)
![lqljwkl/Iff
lqljw+vn' /f]uljz]iflj1fg+ejlttByf
cfKtf]kb]zMk|ToIfd cg'dfg+ r]lt..
r=lj=$.!
rt'lj{wf kl/Iff
t:ort'lj{wfkl/Iff cfKtf]kb]zMk|ToIfd cg'dfg+ o'lQmZr]lt..
r=;'=!!.!&](https://image.slidesharecdn.com/clinicalresearchprotocol-160623145316/85/Clinical-research-protocol-55-320.jpg)
![bzljw k/LIo ljifo
sf/0f – s/0f –sfo{of]gL–sfo{–sfo{kmnfg'aGw–b]z–sfn–
k|j[To'kfofg ;Doulelgj{t{dfgMsfof{lelgj[Qflji6kmnfg'aGw+
sfo{dlelgj{t{oTogltdxtfoTg]gstf{.. r=lj=*.^*
bzljw /f]lukl/Iff
t:dfbft'/+ k/LIf]t k|s[lttZrljs[ltZr;f/tZr ;+xggtZr k|df0ftZr
;fTDotZr ;TjtZrcfxf/zlQmtZrJofofdzlStZr jo:tZr]lt
ank|df0fljz]ifu|x0fx]tf]M.. r=lj=*.($](https://image.slidesharecdn.com/clinicalresearchprotocol-160623145316/85/Clinical-research-protocol-56-320.jpg)









![PROTOCOL AGREEMENT
I have read the protocol specified below. In my formal capacity as Investigator,
my duties include ensuring the safety of the study subjects enrolled under my
supervision and providing [Sponsor Name] with complete and timely
information, as outlined in the protocol. It is understood that all information
pertaining to the study will be held strictly confidential and that this
confidentiality requirement applies to all study staff at this site. Furthermore, on
behalf of the study staff and myself, I agree to maintain the procedures required
to carry out the study in accordance with accepted GCP principles and to abide
by the terms of this protocol.
Protocol Number: Number
Protocol Title: Title
Protocol Date: TBD
Investigator Signature Date
Print Name and Title
Site #
Site Name
Address](https://image.slidesharecdn.com/clinicalresearchprotocol-160623145316/85/Clinical-research-protocol-67-320.jpg)