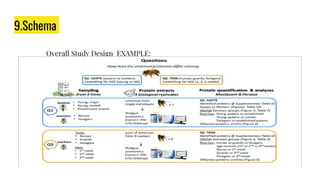
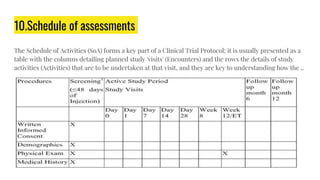
The document outlines a clinical study protocol, detailing the plan for conducting a clinical trial while adhering to ethical guidelines and ensuring participant safety. Key components include study objectives, design, inclusion/exclusion criteria, safety measures, schema, and schedules of assessments. It serves as a comprehensive guide for stakeholders involved in the trial, including investigators, sponsors, and regulatory bodies.