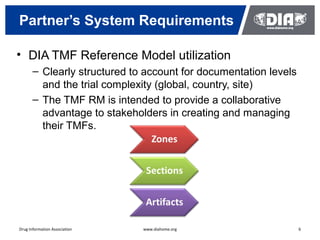
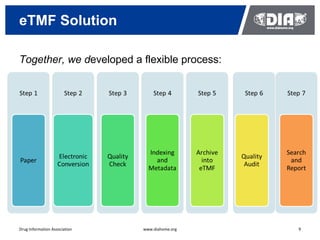
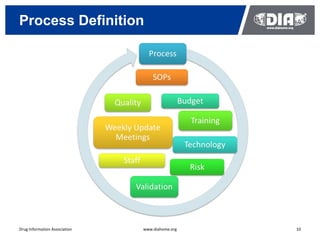
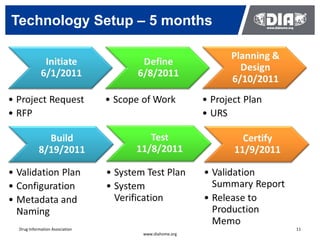
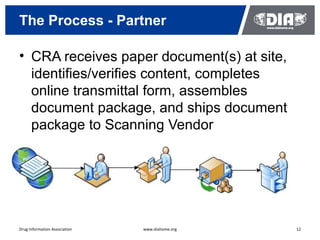
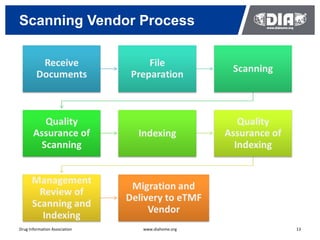
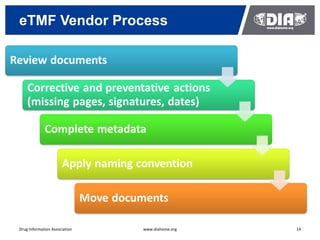
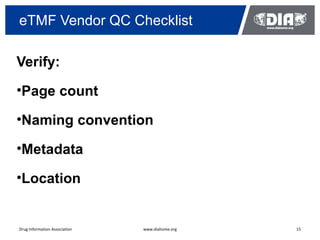
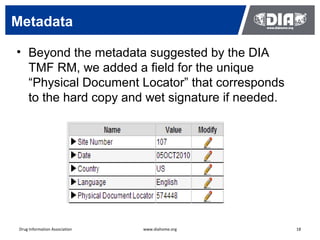
The document presents a case study on the implementation of an electronic trial master file (eTMF) system at a biotech company aiming to streamline processes for a pivotal phase 3 clinical study. It details challenges with paper-based documentation, objectives for resource optimization, and the steps taken to transition to a cloud-based solution. Key outcomes include improved accessibility of study records, enhanced TMF quality, and reduced costs associated with document management.