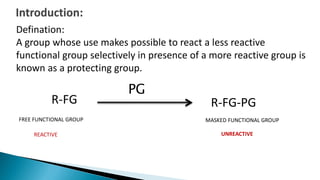
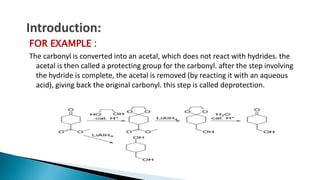
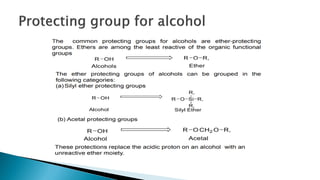
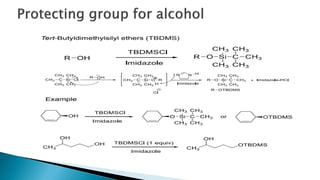
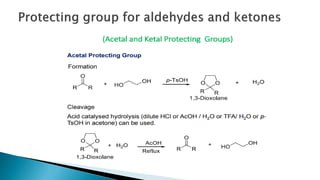
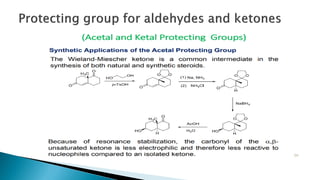
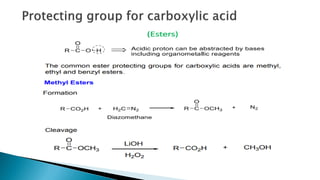
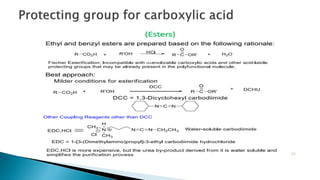
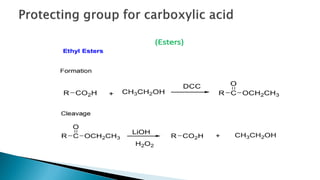
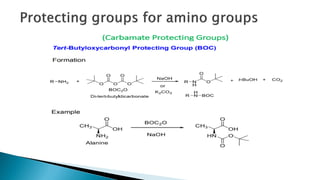
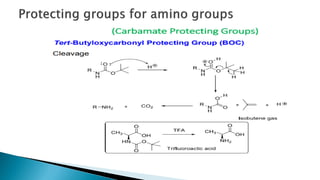
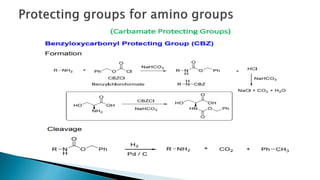
The document discusses protecting groups in organic synthesis, which are used to mask the reactivity of functional groups to enable selective reactions. Key qualities of effective protecting groups include ease of introduction, stability during reactions, and ability to be removed under mild conditions. Examples and references are provided to illustrate the importance of protecting groups in synthesizing complex molecules.