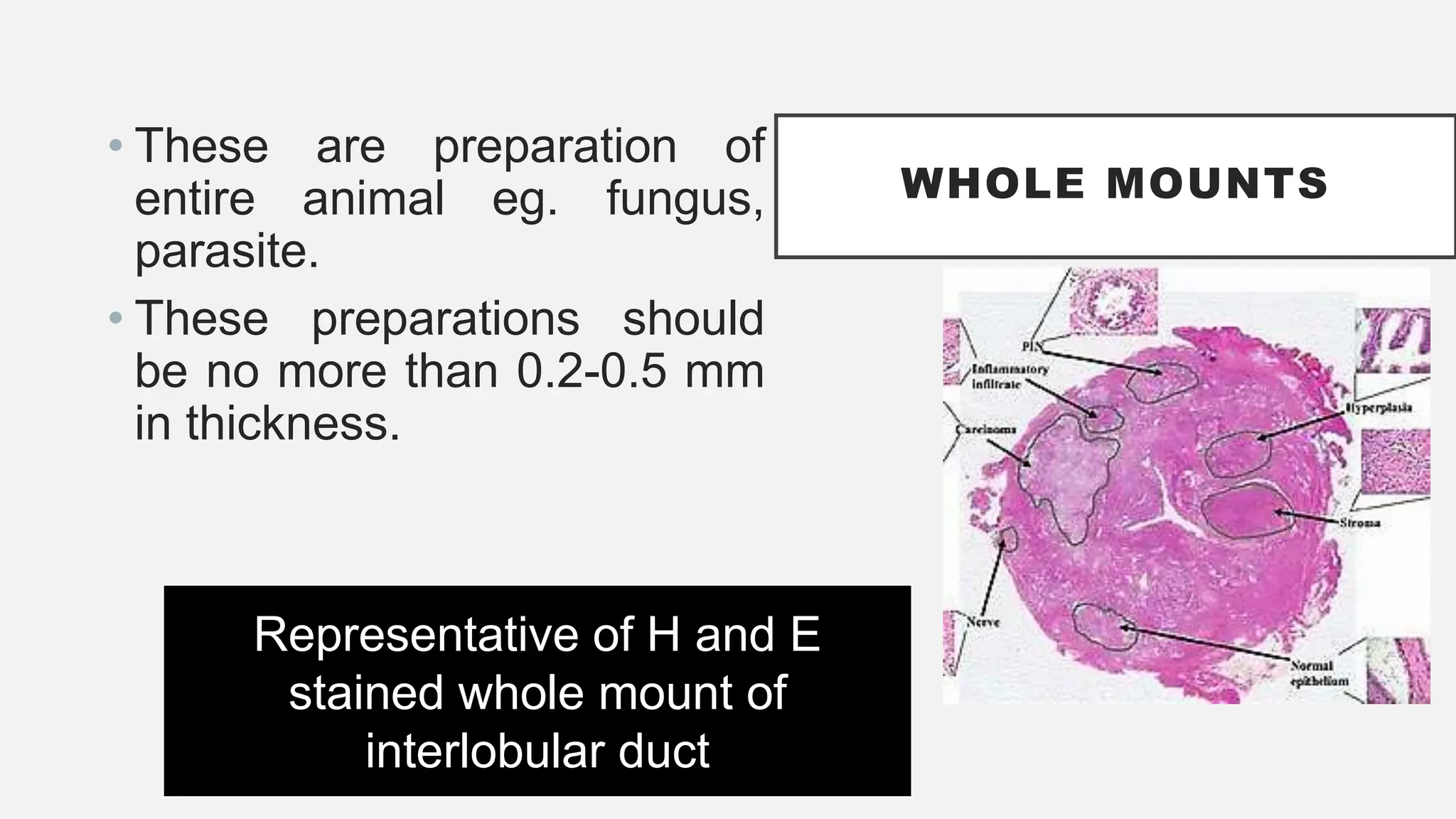
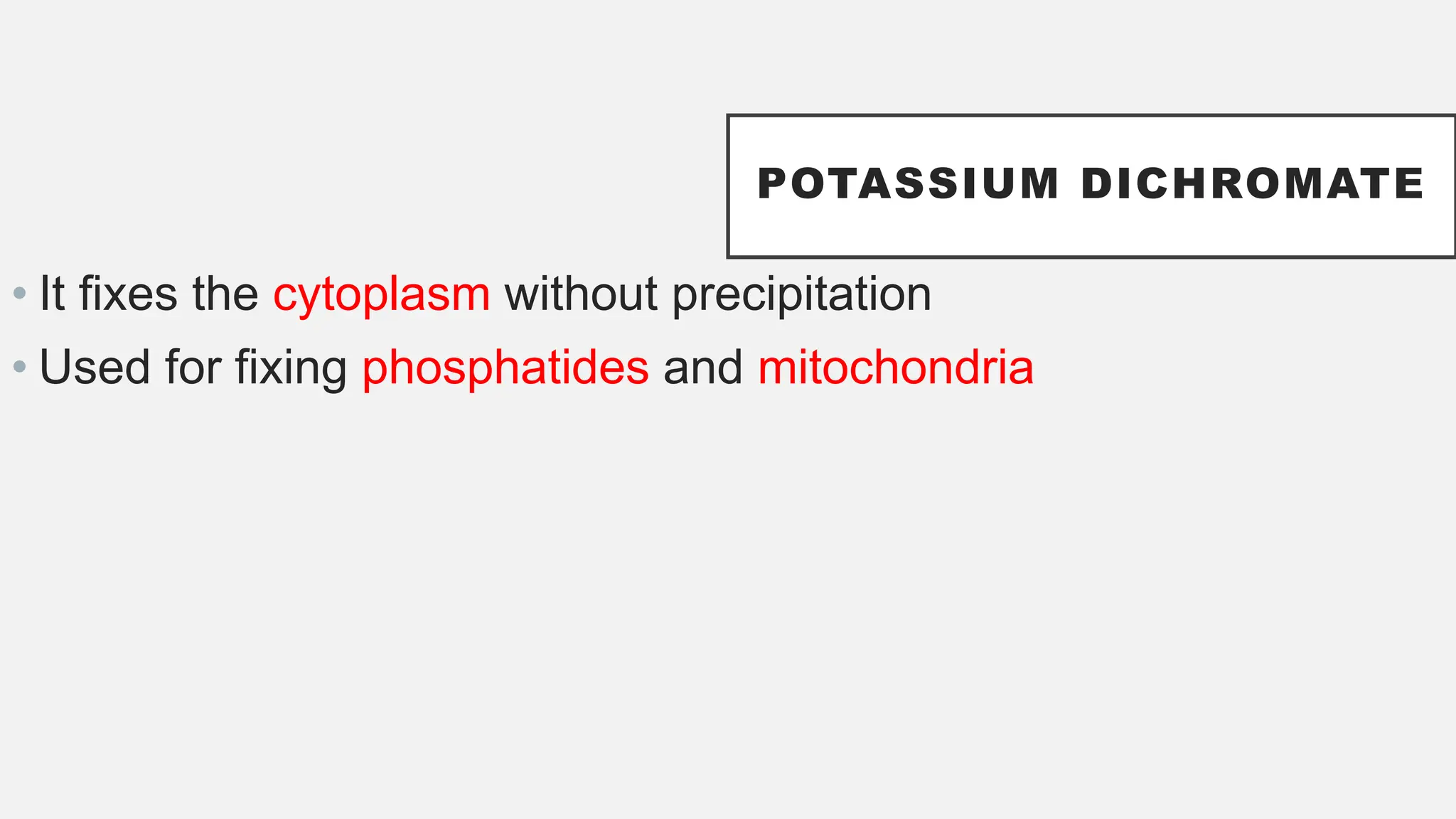
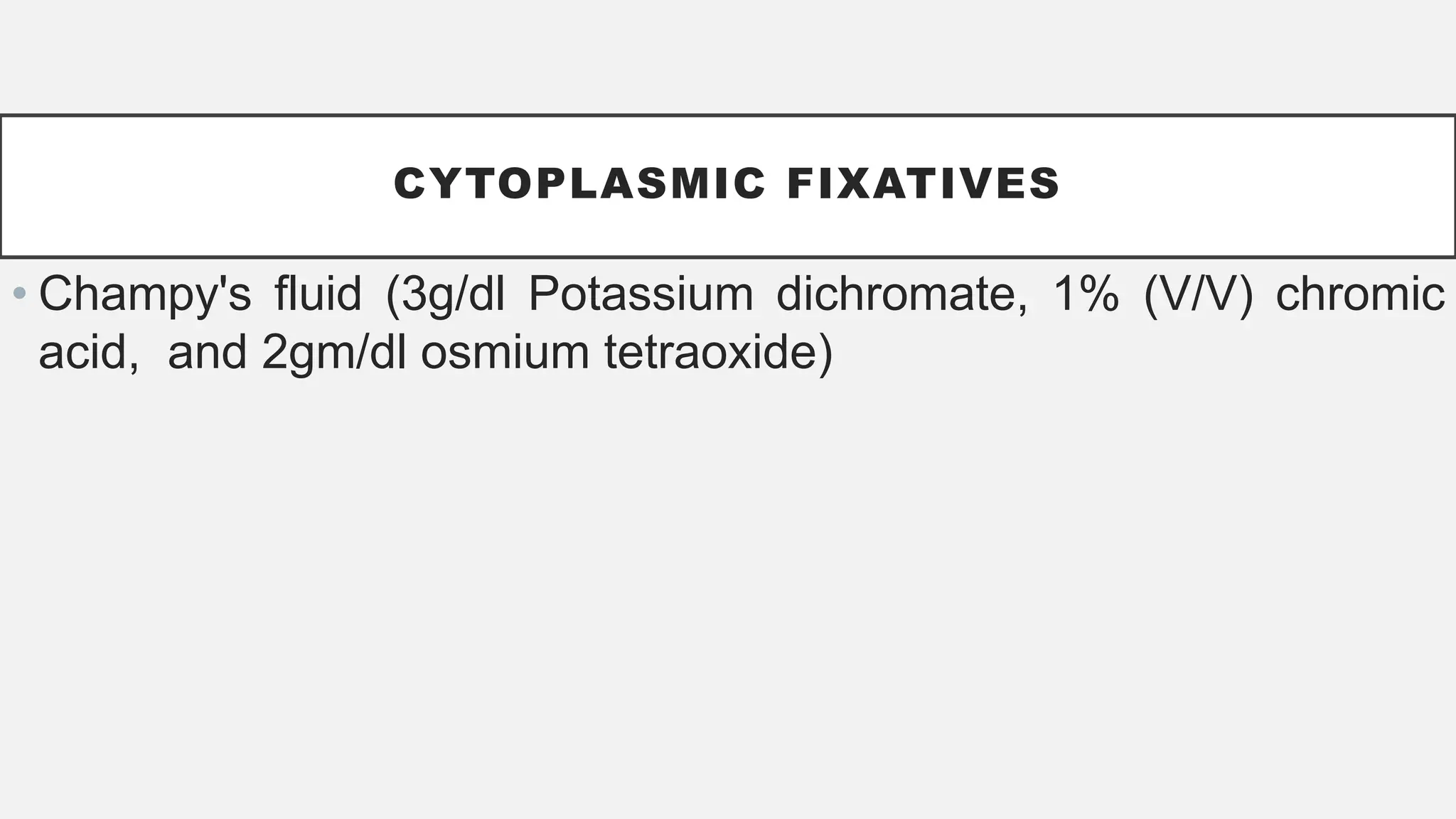
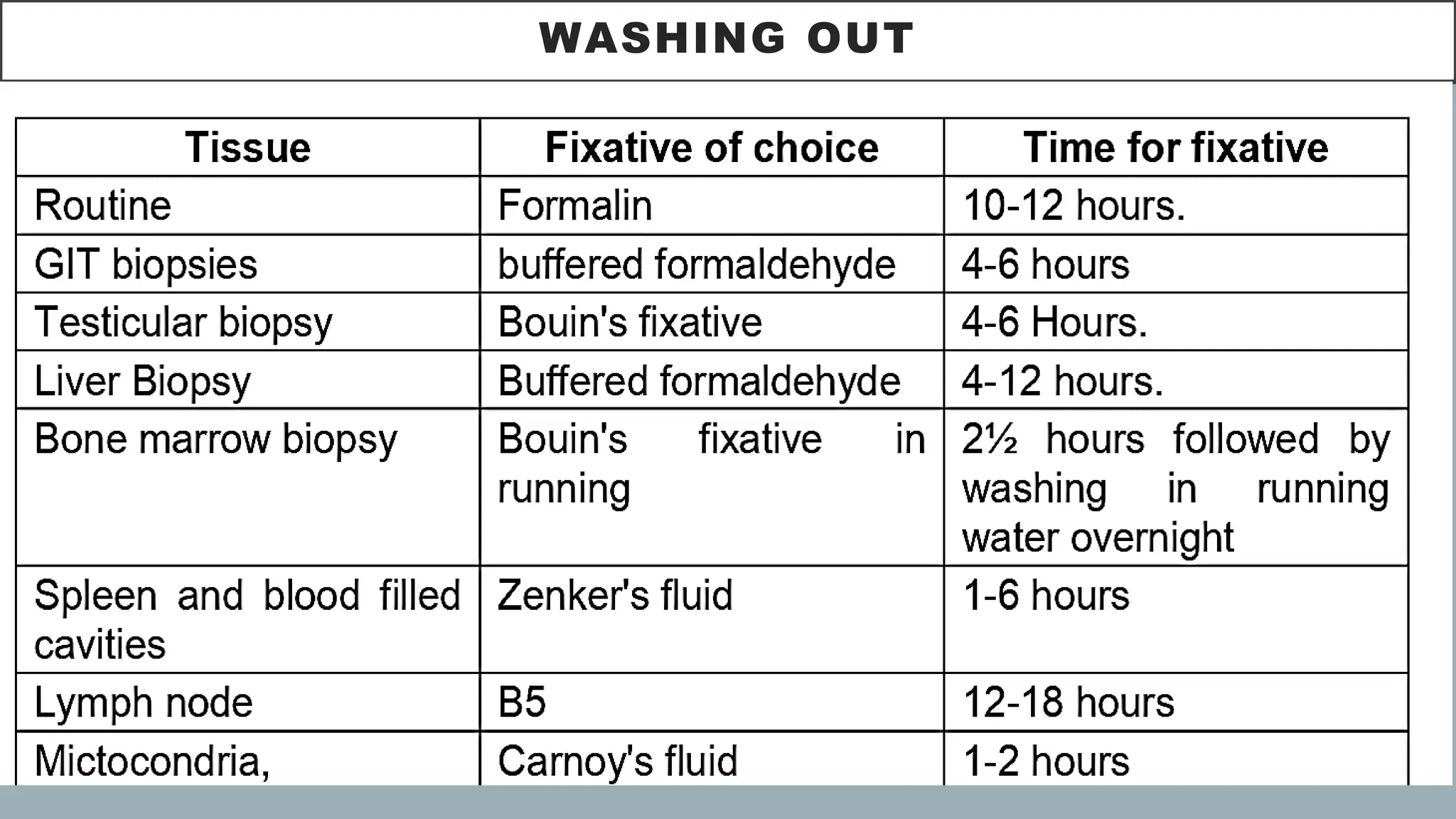
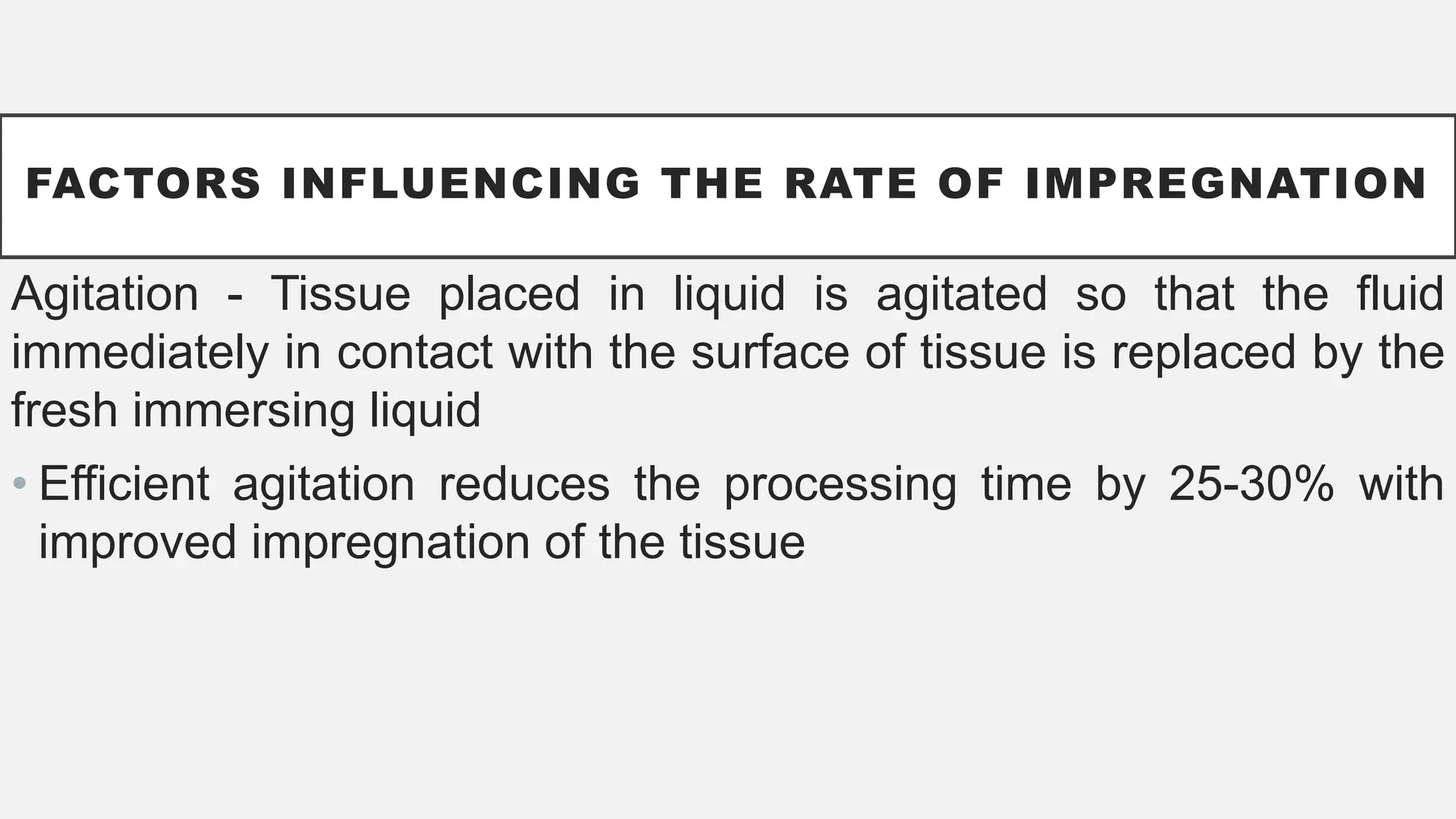
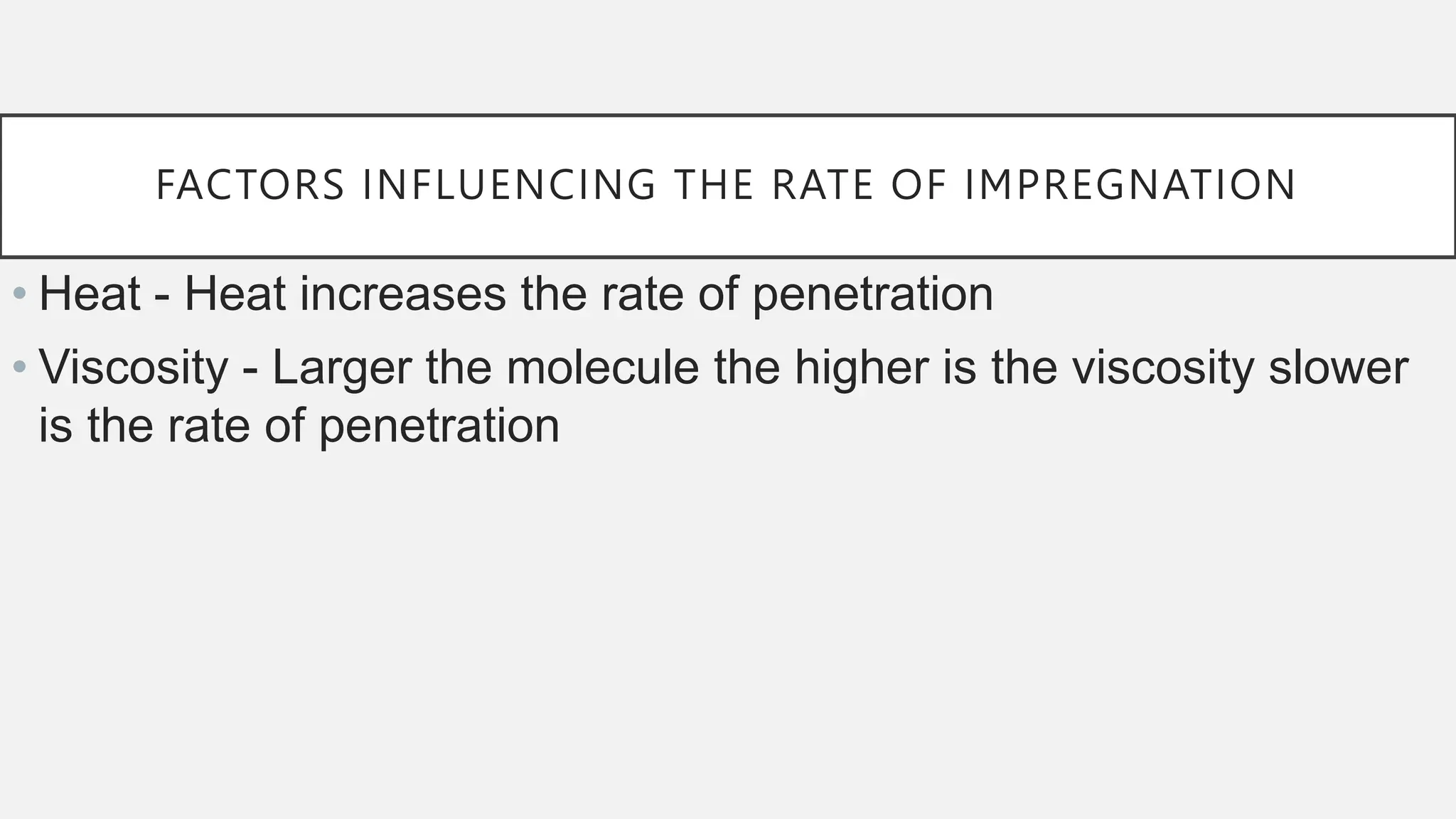
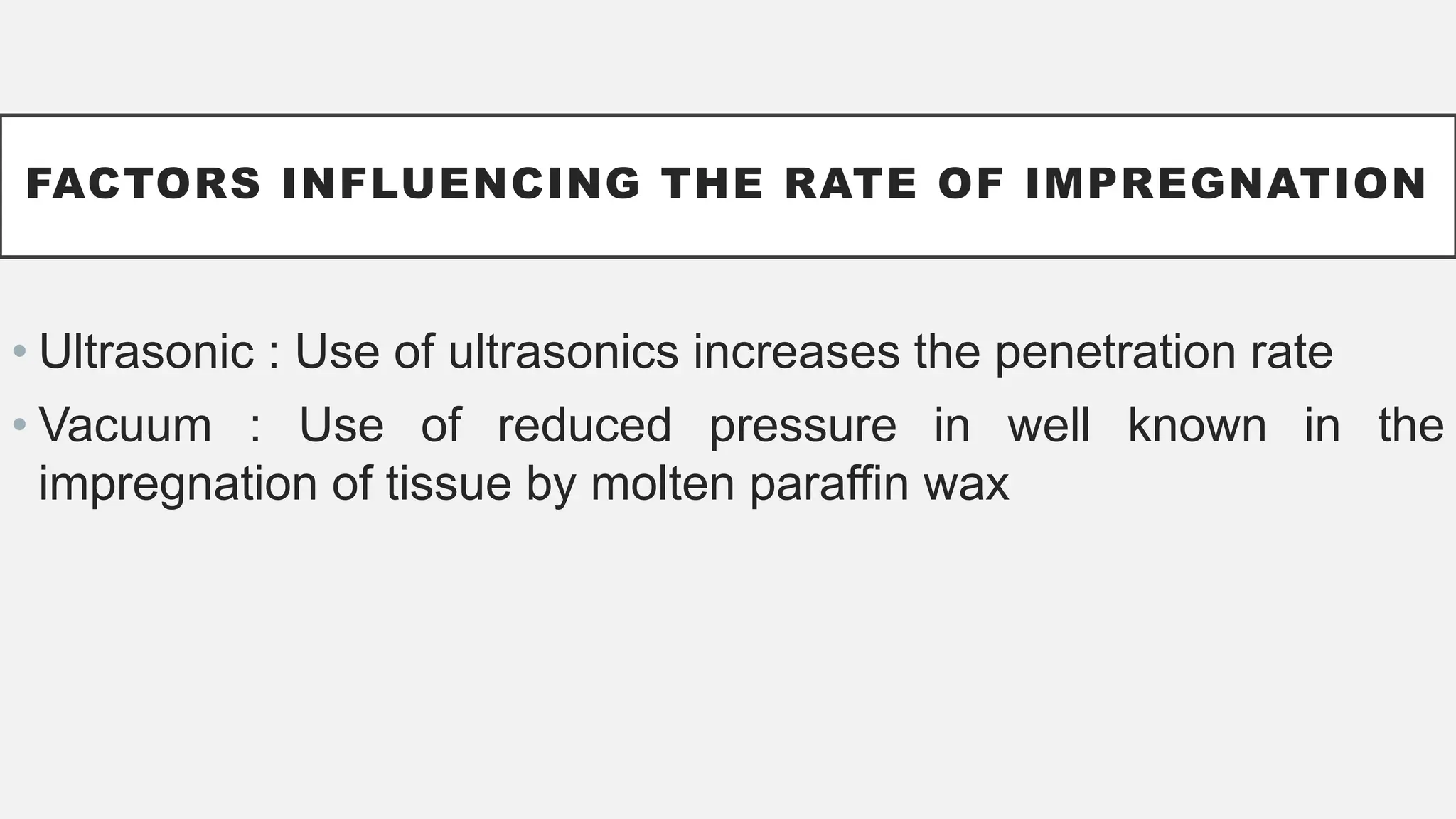
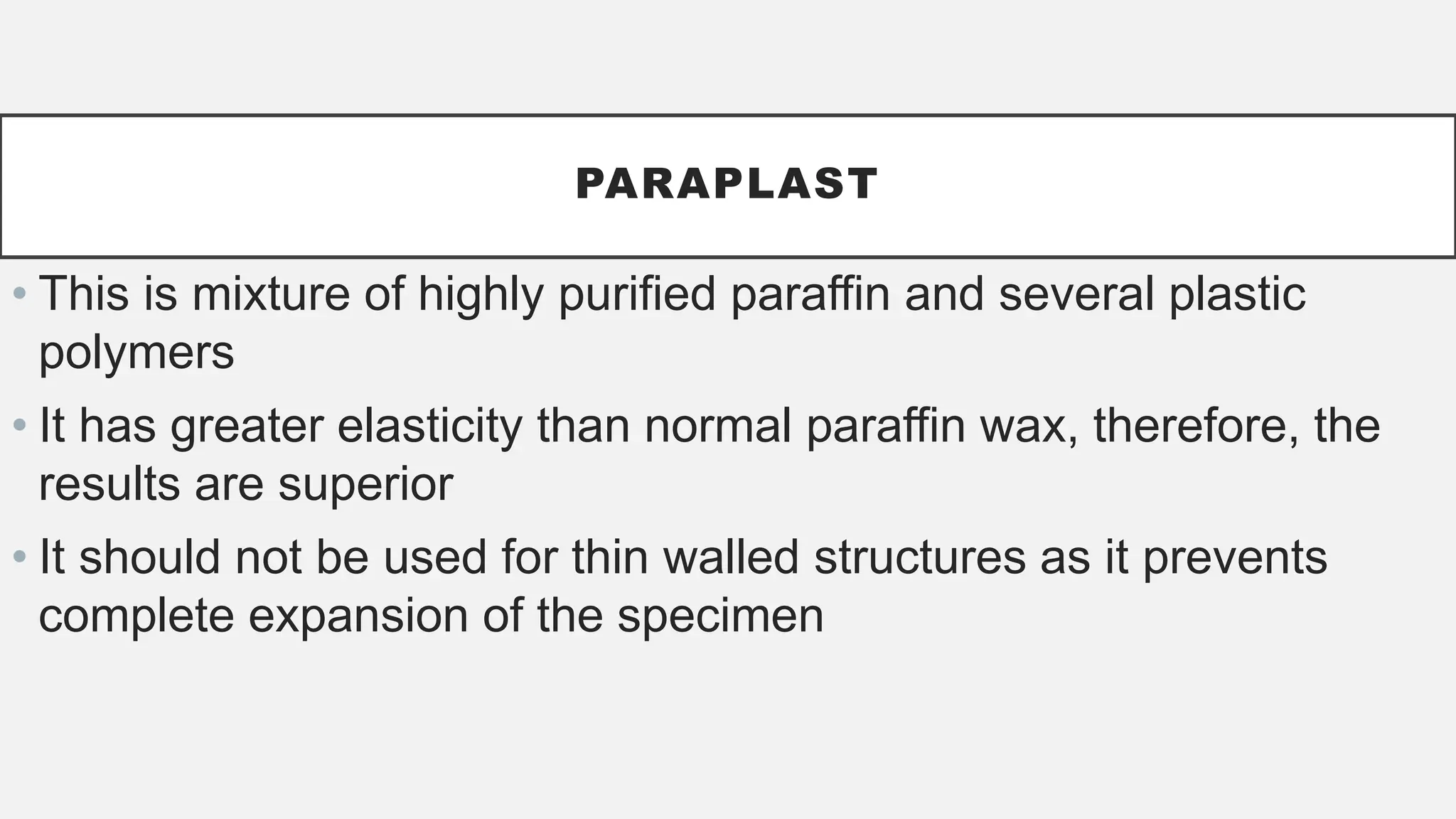
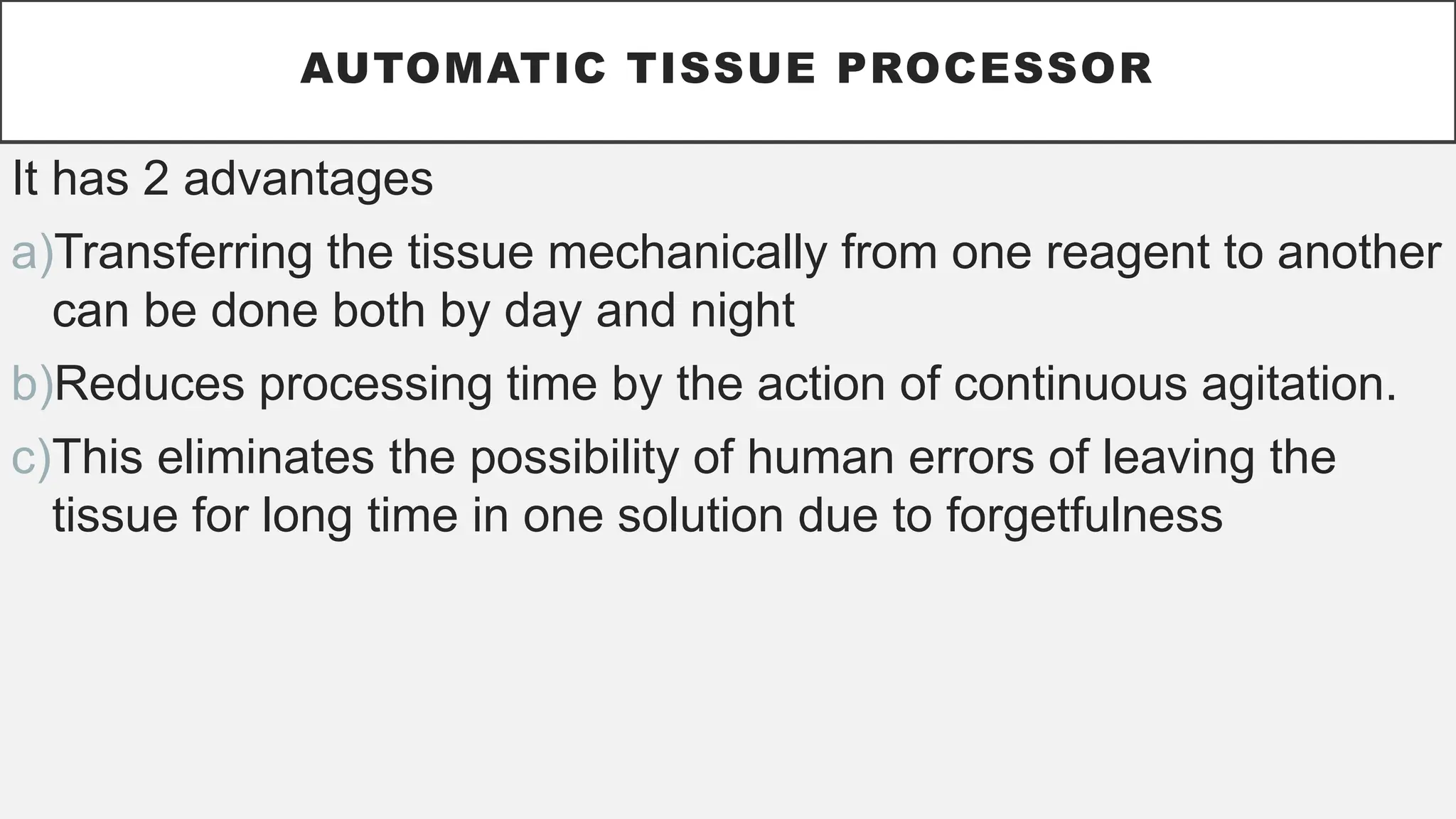
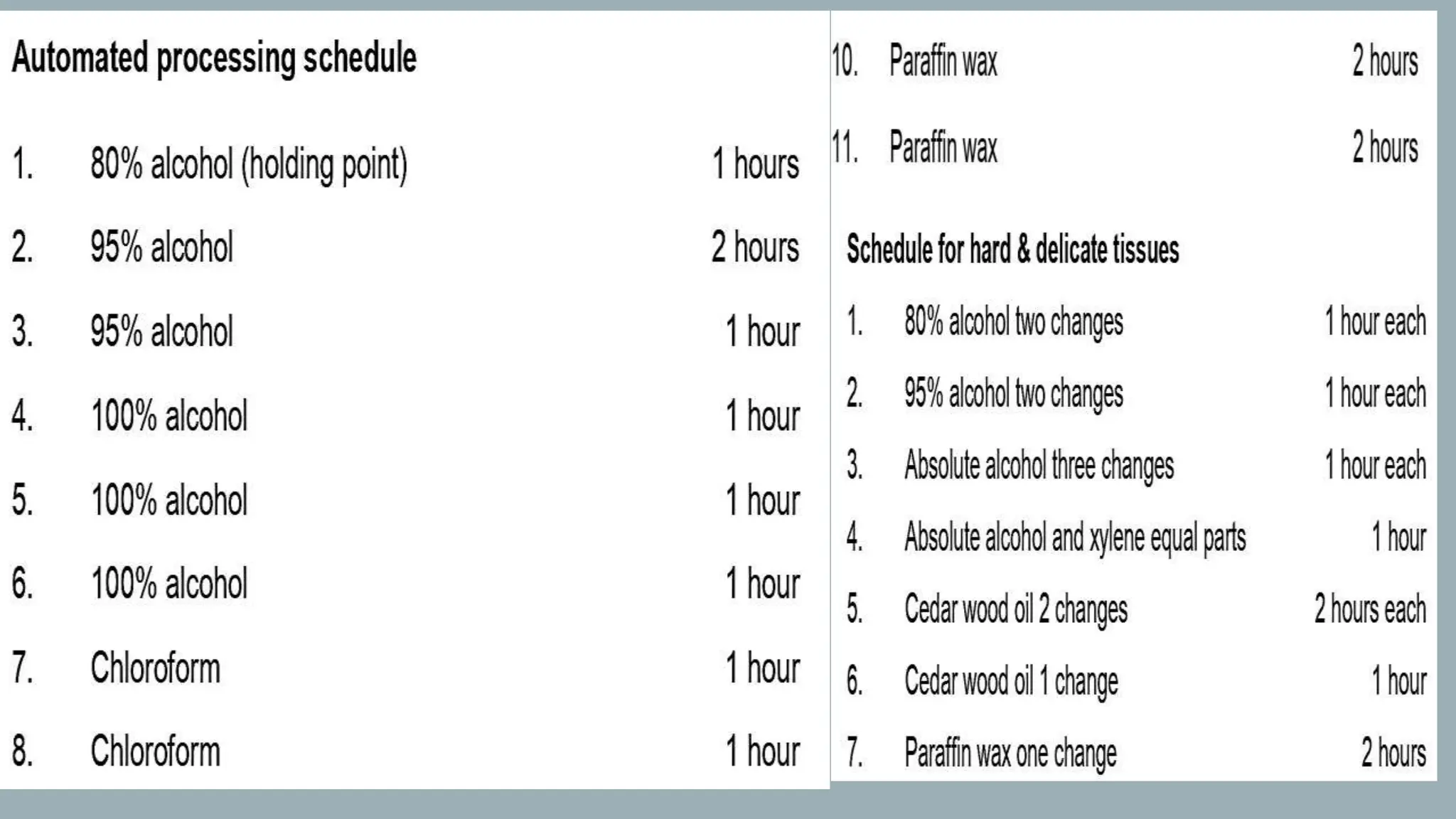
The document presents an overview of histopathology, including techniques for tissue handling, processing, and staining. It describes types of histological preparations such as whole mounts, sections, and smears, and outlines the principles and methods of fixation, dehydration, and embedding. Additionally, it explains various fixatives, their properties, and applications in preserving tissue for microscopic examination.