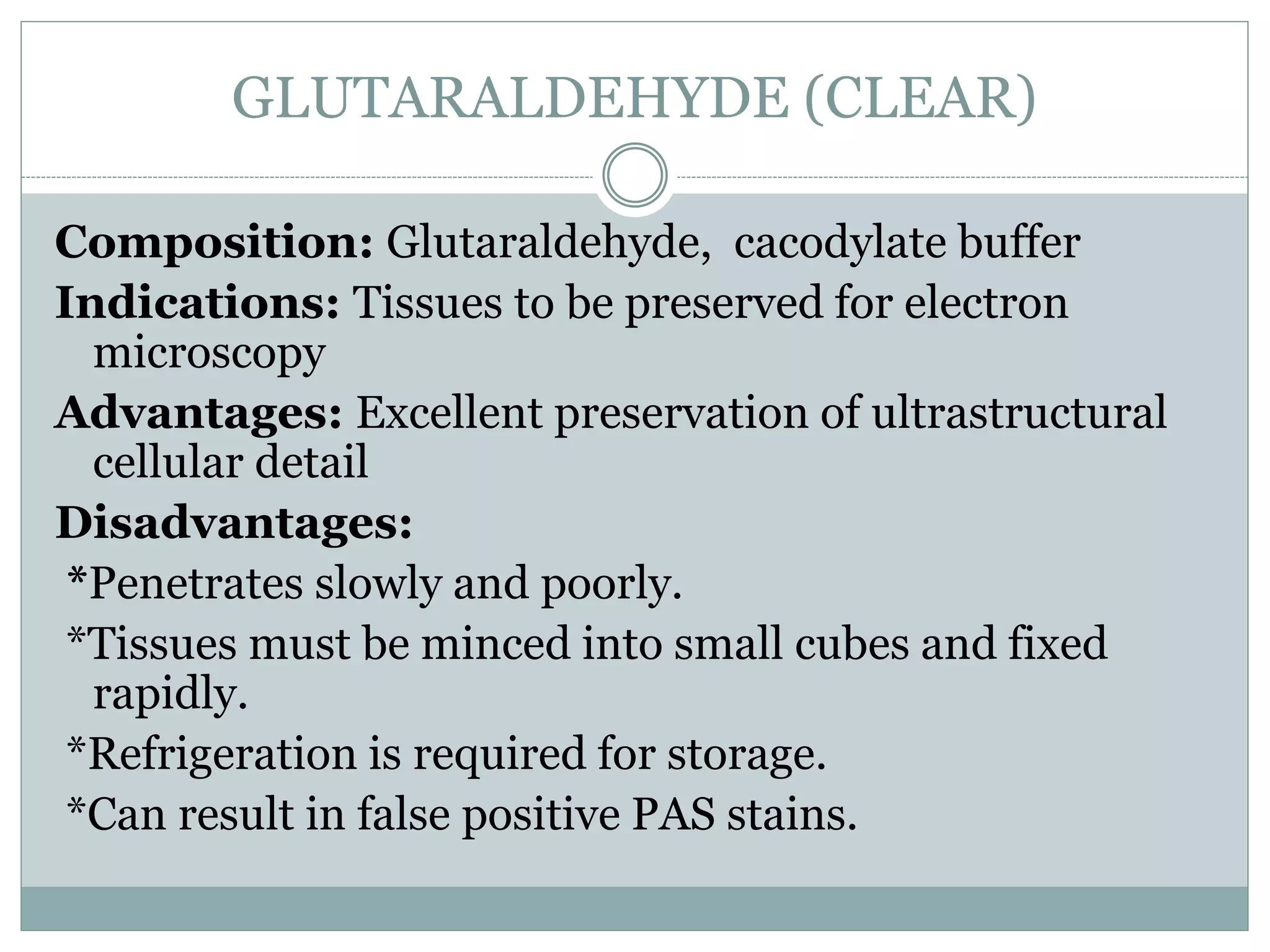
This document discusses various tissue fixation methods used in histopathology. Fixation preserves tissues for examination by hardening them and preventing degradation. The ideal fixative kills and penetrates tissue quickly without shrinkage while stabilizing components and enhancing staining. Formalin is commonly used as it fixes most tissues well and is compatible with many stains, though it causes some shrinkage and nucleic acid degradation over time. Other fixatives include Bouin's solution, B-Plus, Zenker's, alcohol and glutaraldehyde. Proper disposal of fixatives and fixed tissues is also important due to their toxic, infectious or hazardous properties.