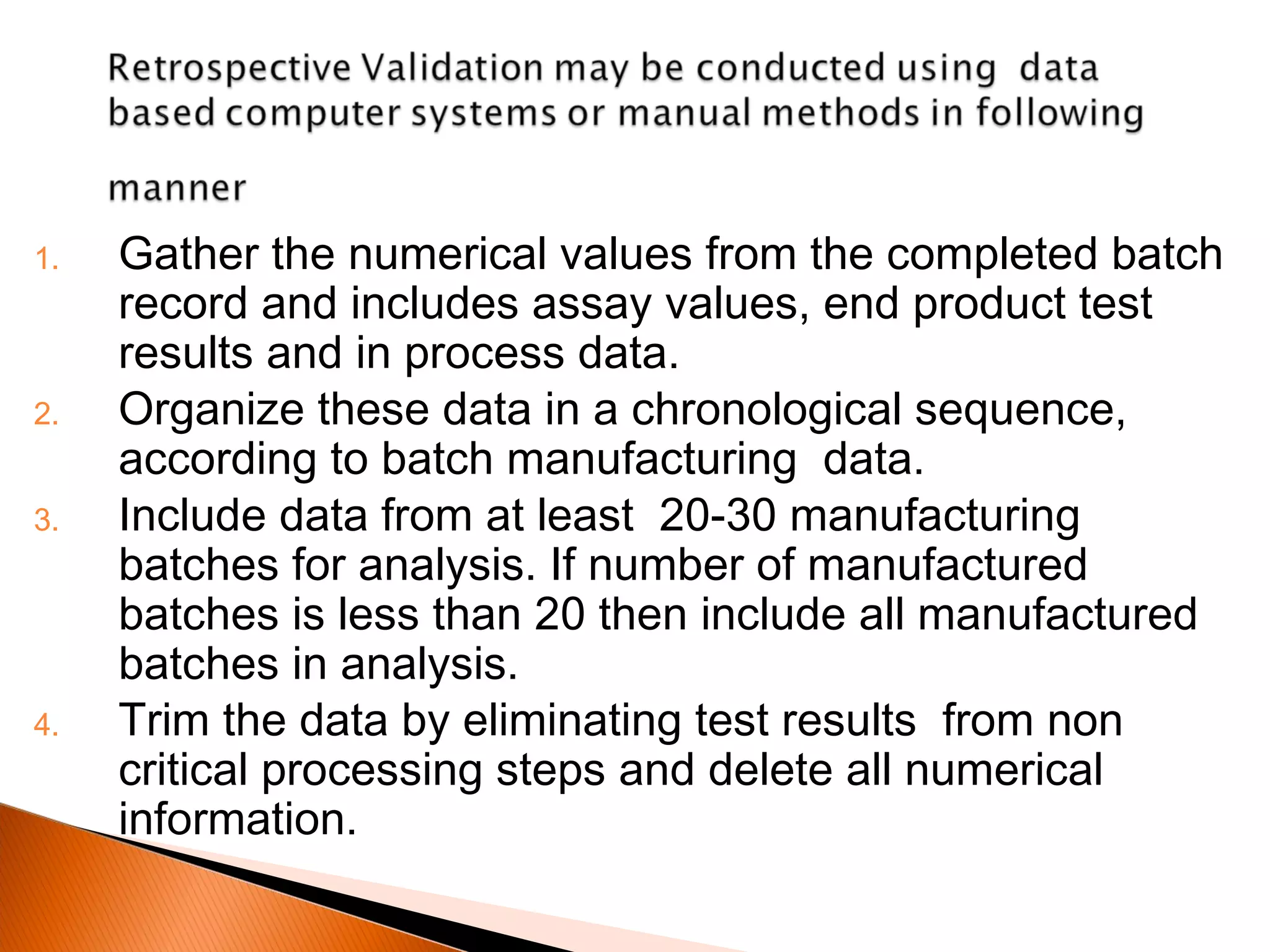
Process validation establishes documented evidence that a manufacturing process will consistently produce products meeting specifications. It involves qualifying facilities and equipment, validating critical process parameters, and revalidating when changes occur. Validation includes prospective validation of new processes and retrospective validation of existing stable processes by statistical analysis of historical batch data. Documentation of the validation master plan, protocols, reports, and results provide assurance that processes are properly controlled.