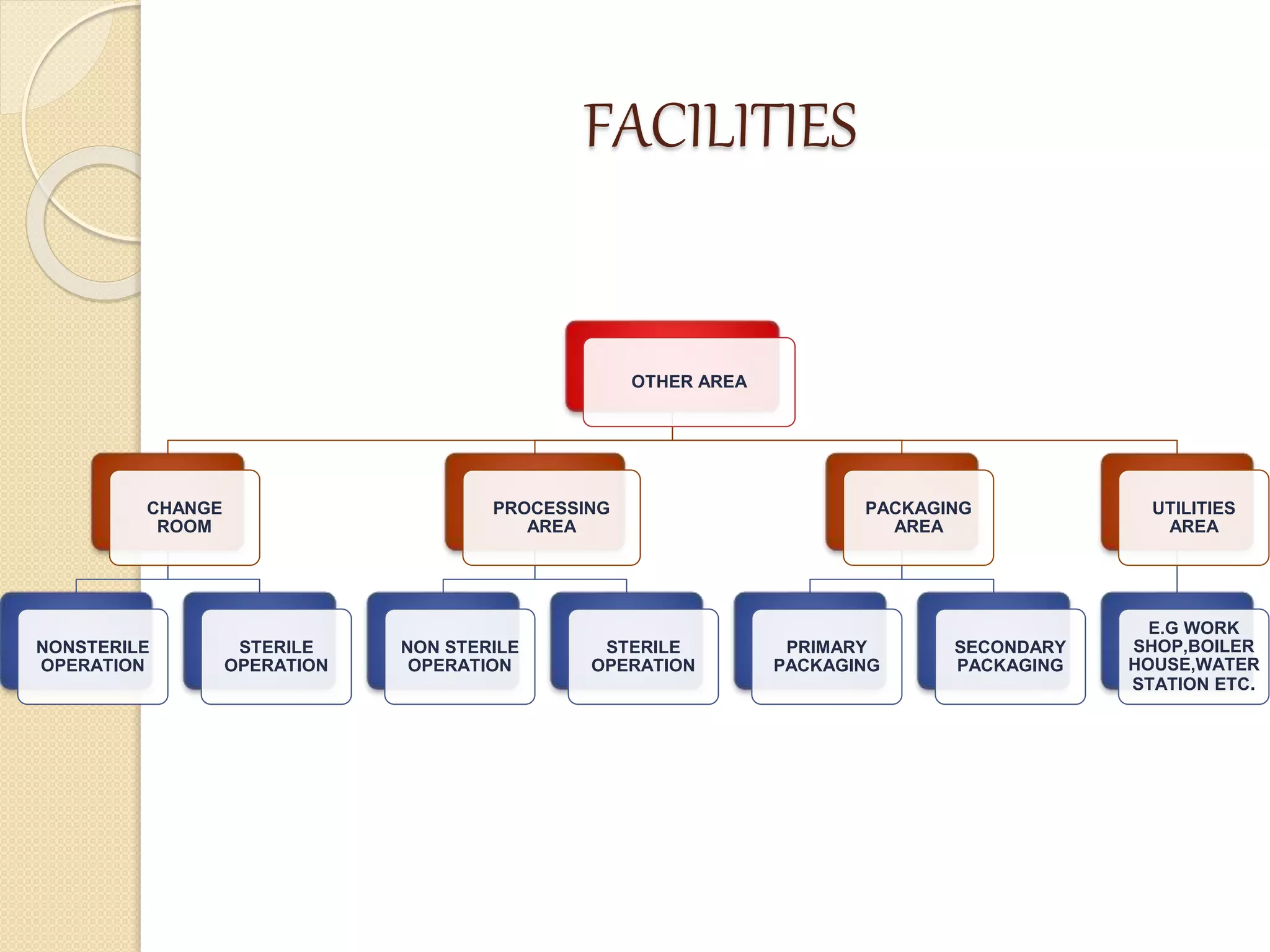
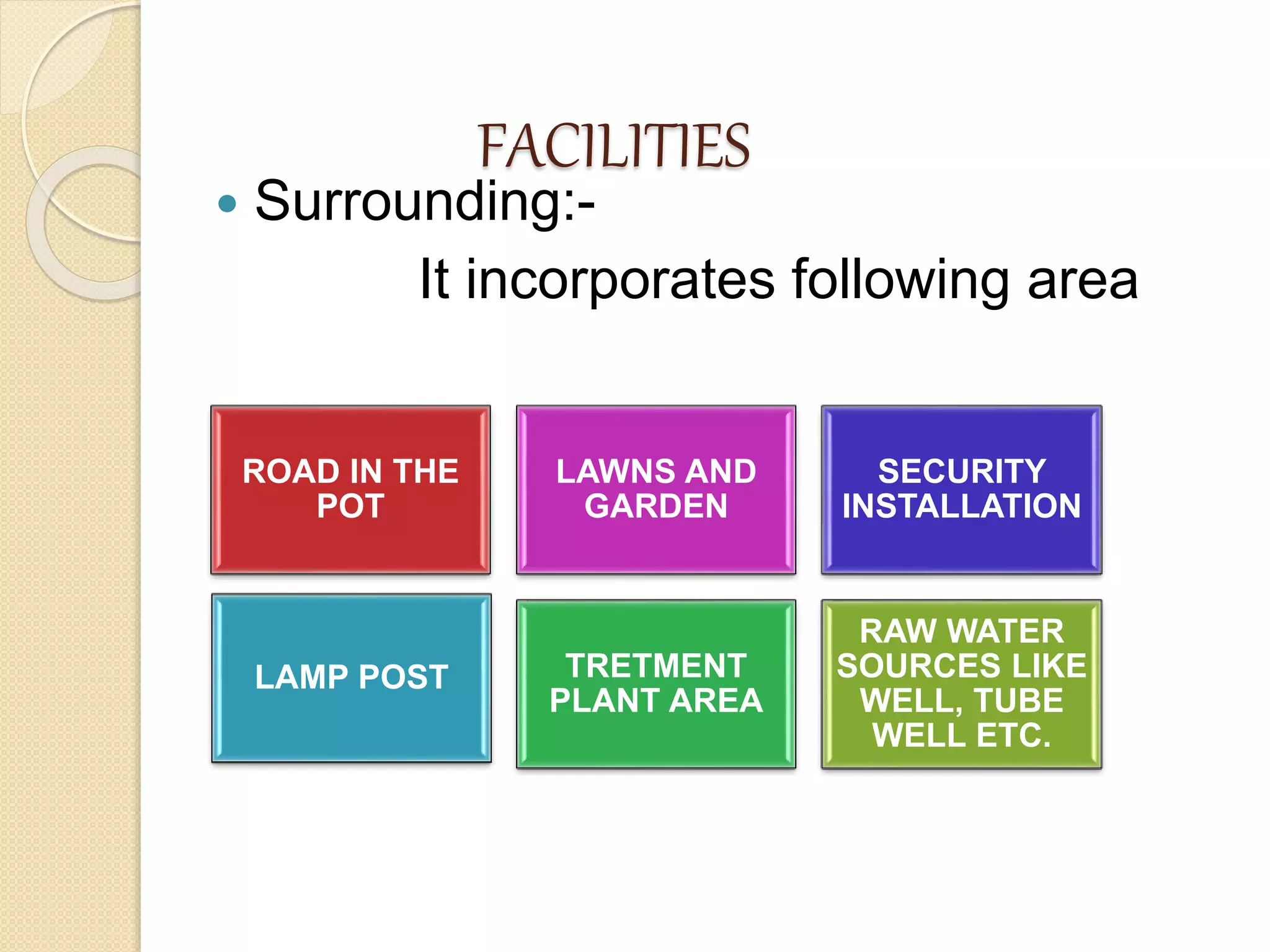
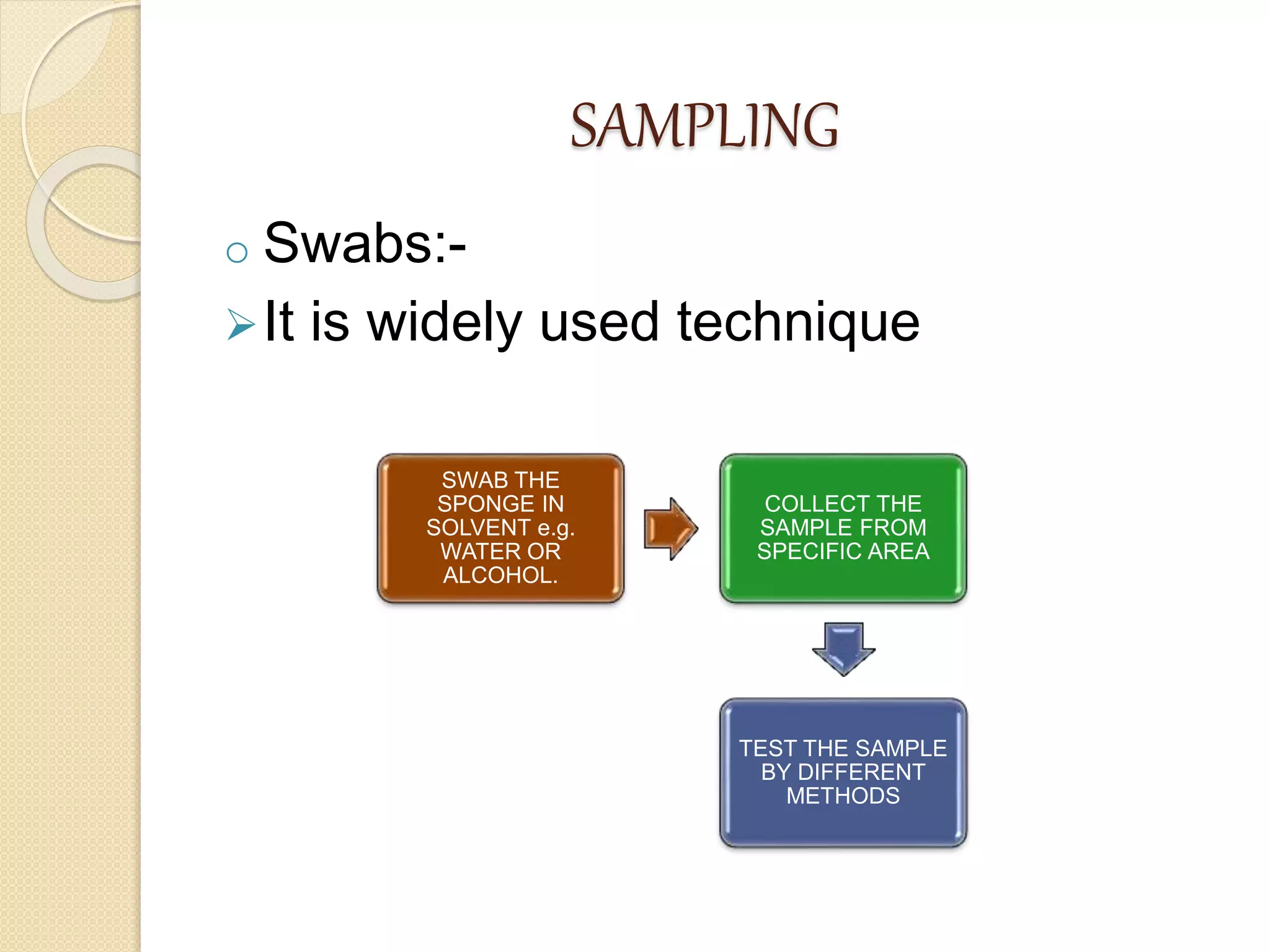
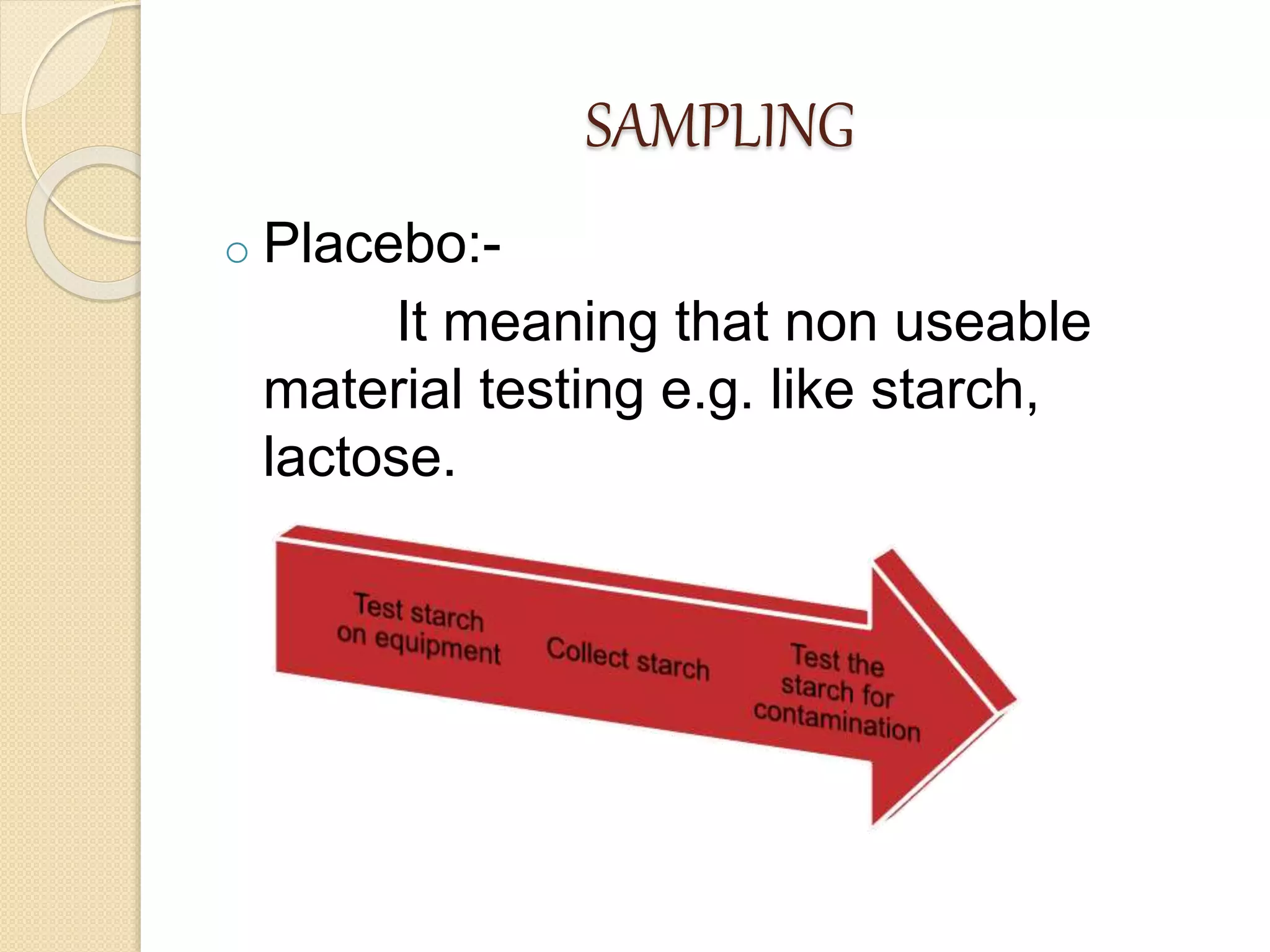
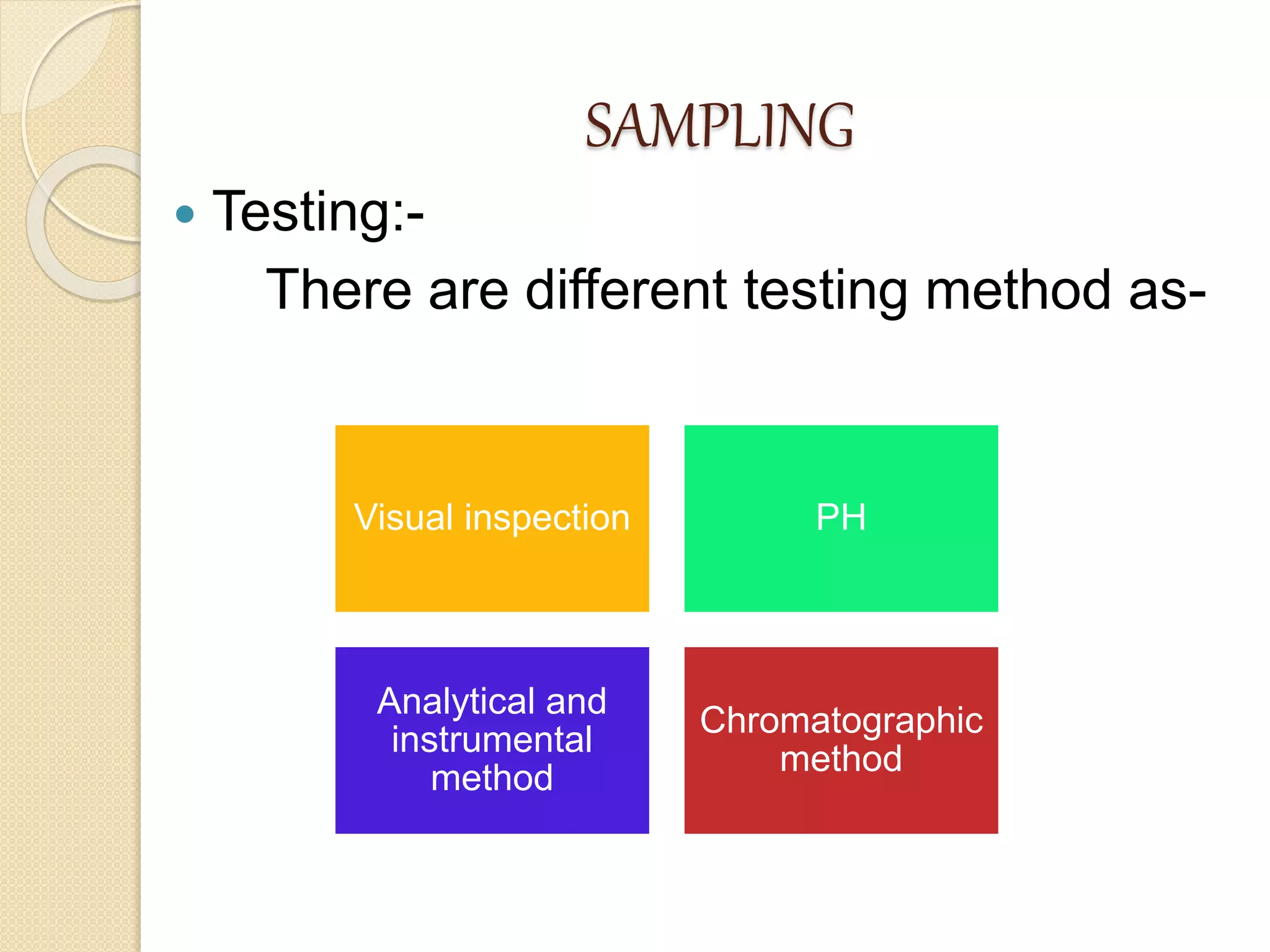
This document discusses cleaning validation in the pharmaceutical industry. It begins by defining cleaning validation and outlining regulatory requirements for cleaning. Key factors in developing a cleaning validation protocol are then summarized, including the product being cleaned, equipment, facilities, cleaning methods, cleaning agents, and sampling and testing. The document stresses that cleaning validation ensures removal of residues and contaminants to meet purity standards for safety. It provides an overview of considerations for each validation factor to help establish effective cleaning procedures.