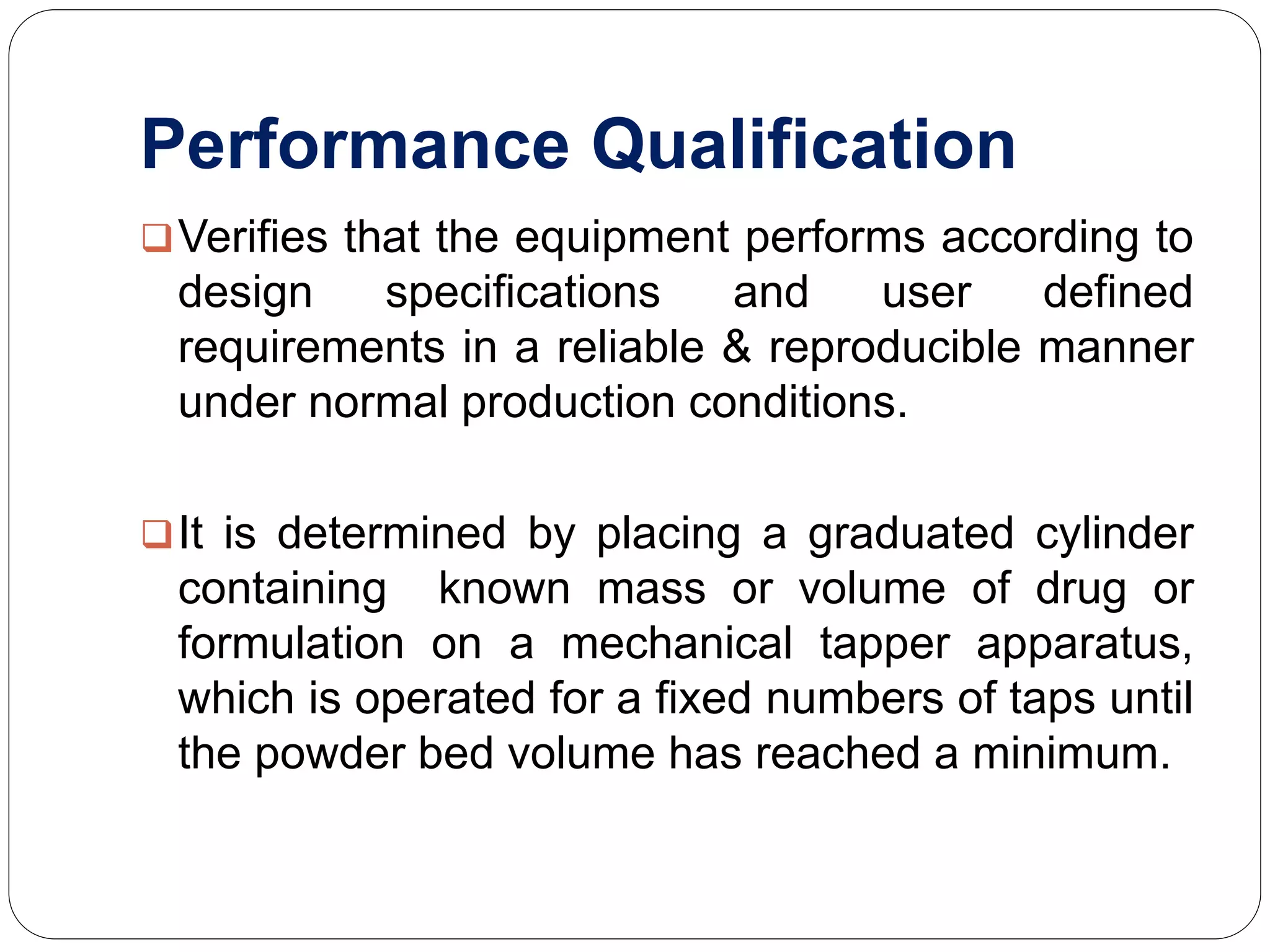
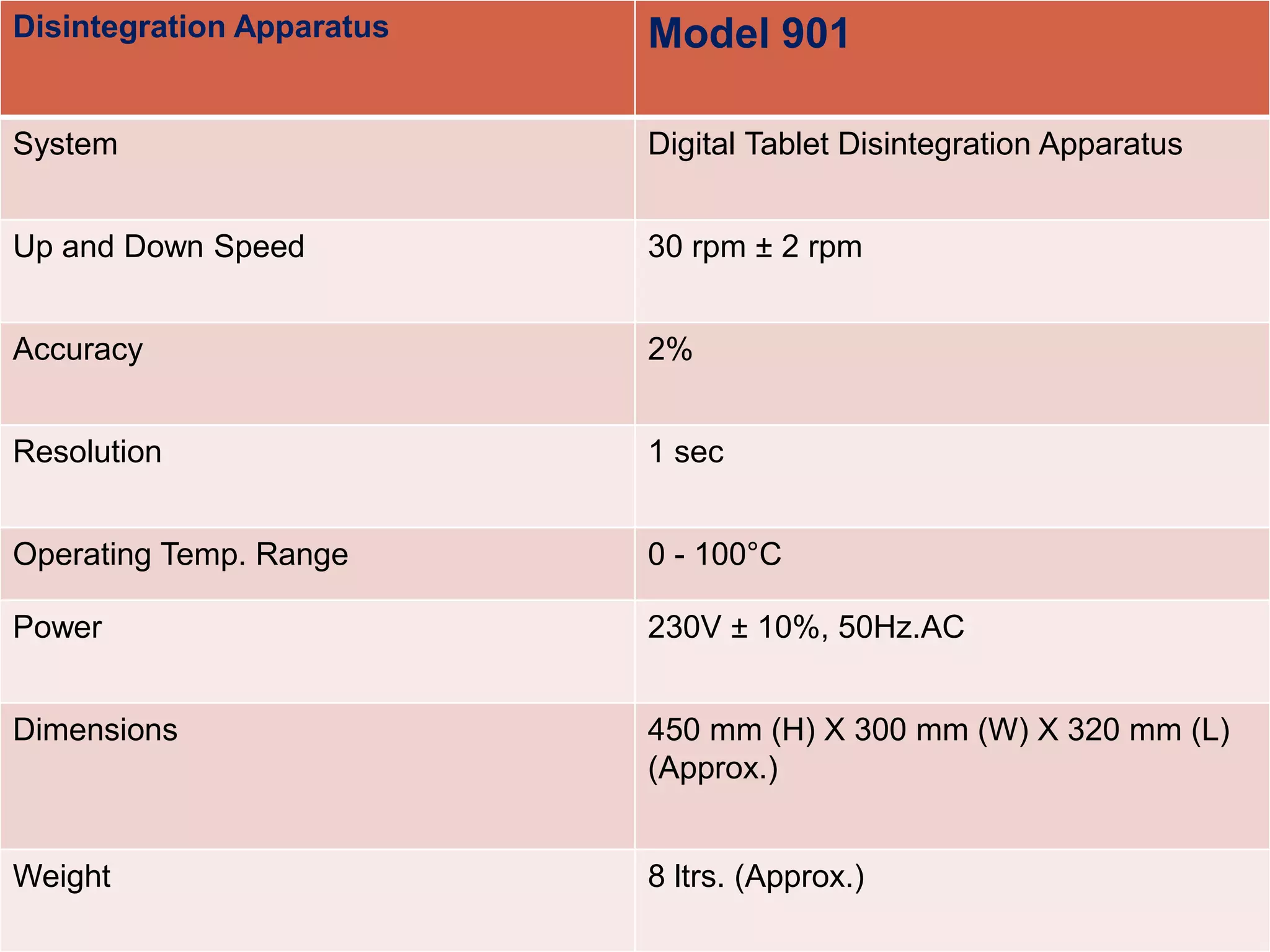
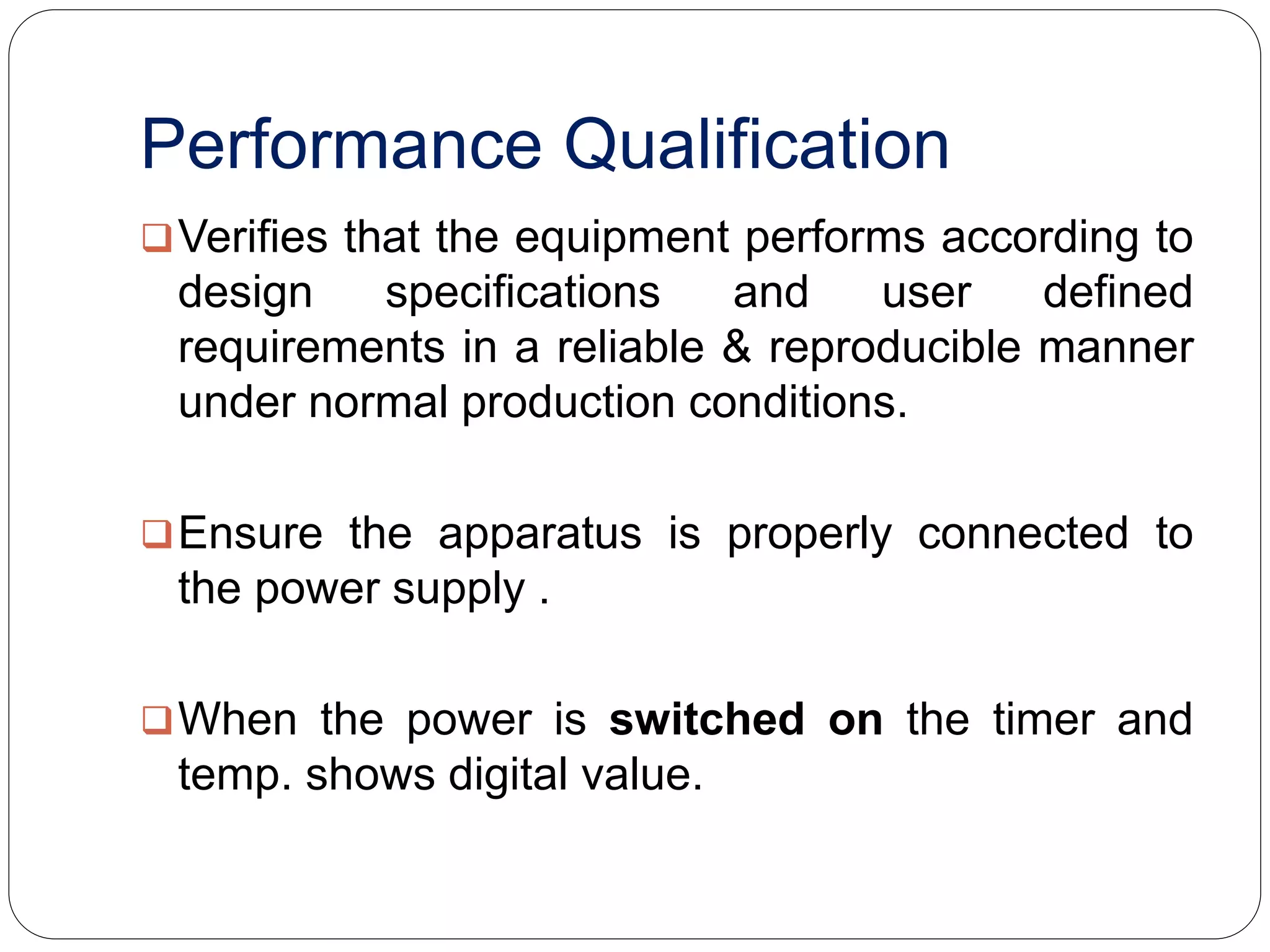
The document discusses the qualification requirements for a tapped density tester and disintegration tester used in pharmaceutical validation. It outlines the user requirements, design qualification, installation qualification, operational qualification, and performance qualification that must be completed to ensure the equipment is properly installed and operating as intended according to specifications. Key steps include verifying the equipment dimensions and operating conditions, testing that the tapped density tester can accurately measure density and the disintegration tester can oscillate tablets at the appropriate speed and temperature.