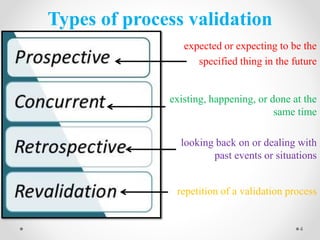
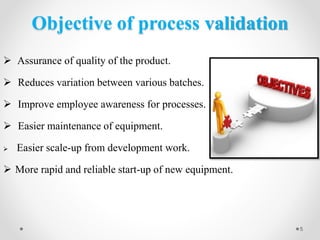
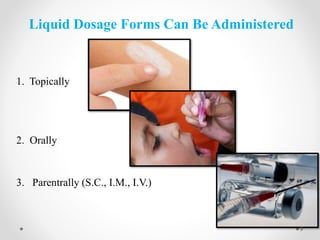
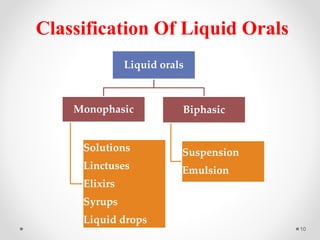
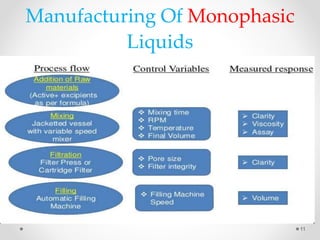
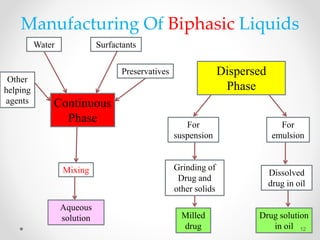
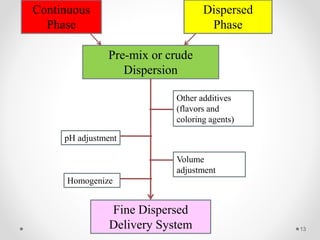
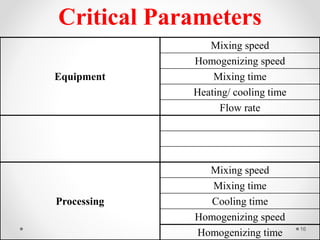
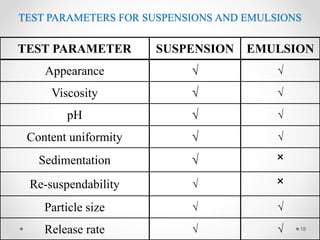
This document discusses process validation for liquid oral dosage forms. It defines process validation and explains that it ensures consistent production of products meeting quality standards. The objectives are to assure product quality and reduce batch variation. Types of liquid orals include solutions, suspensions, and emulsions. Critical process parameters for equipment, processing, and acceptance criteria are identified. The validation operations described include testing of raw materials and monitoring of outputs like appearance, pH, and content uniformity. A validation report is prepared and changes may require revalidation.