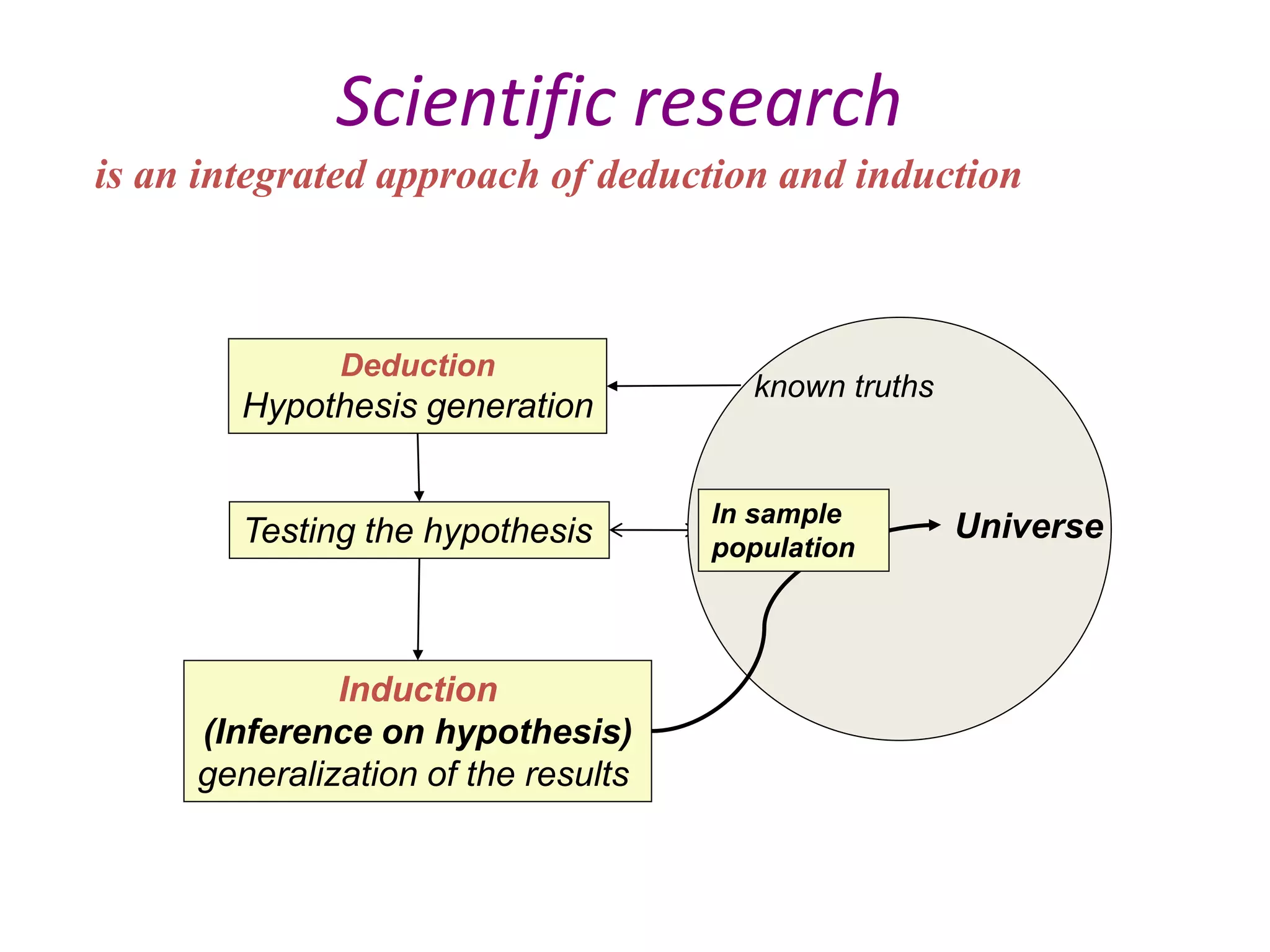
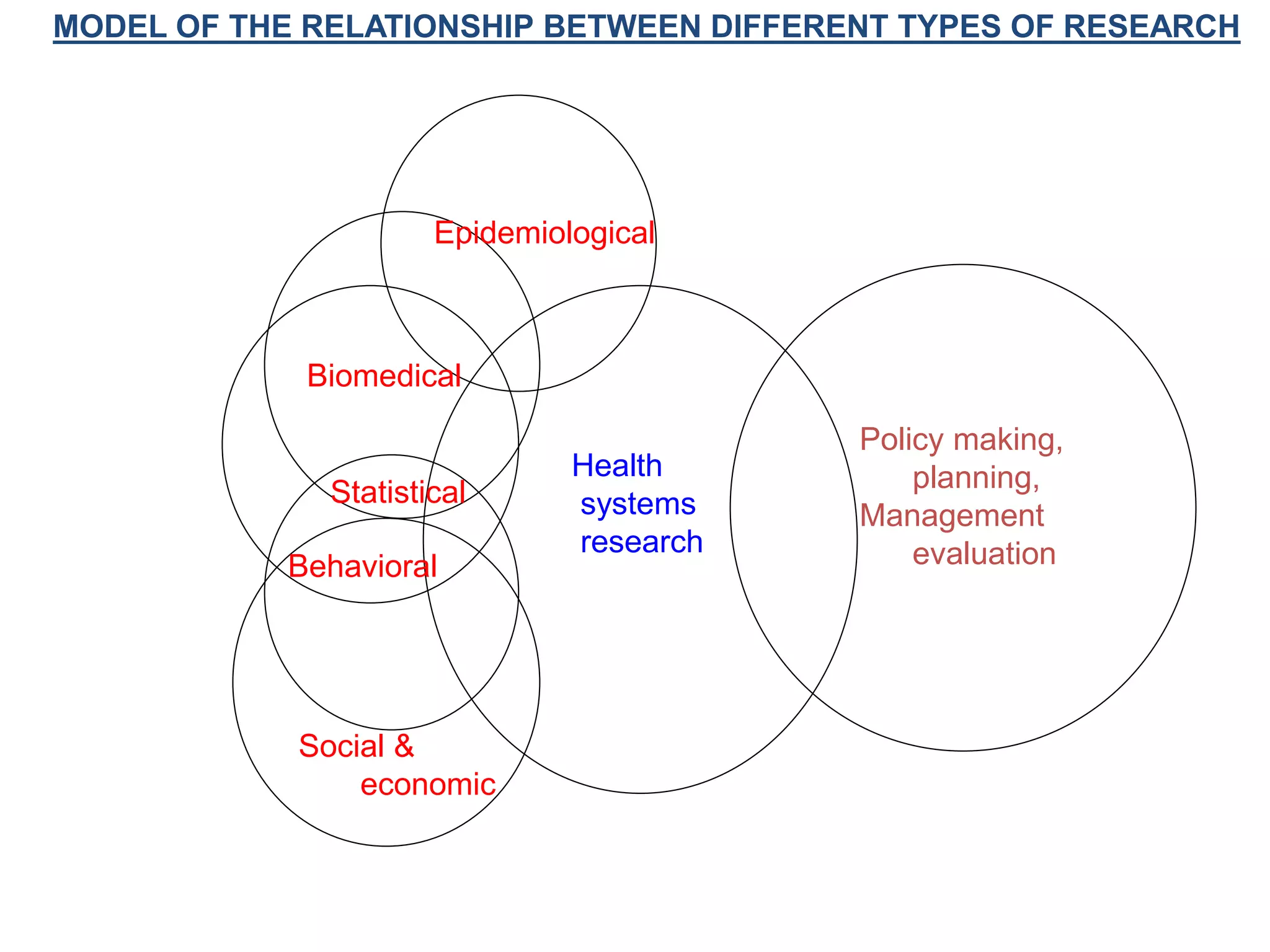
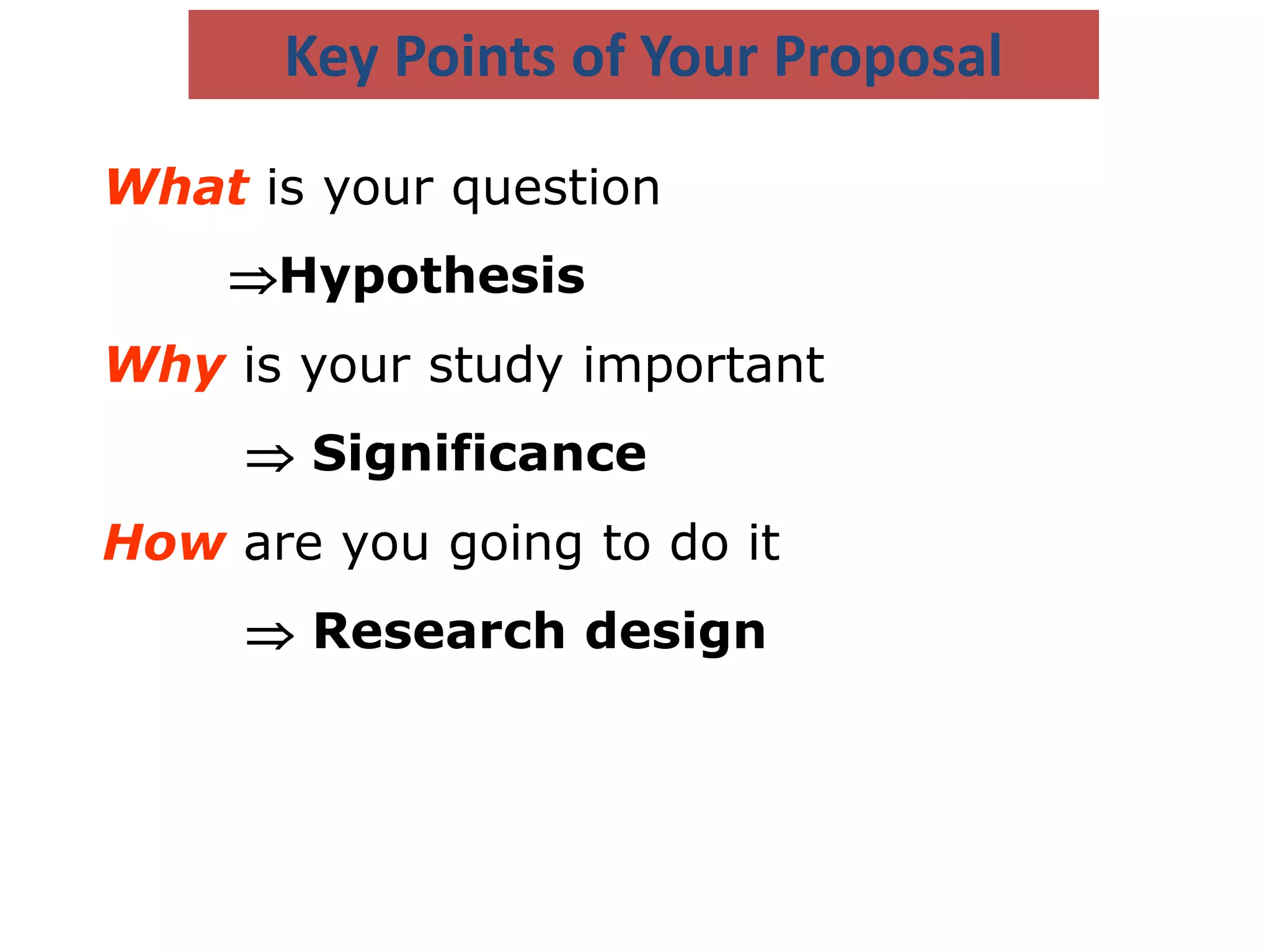
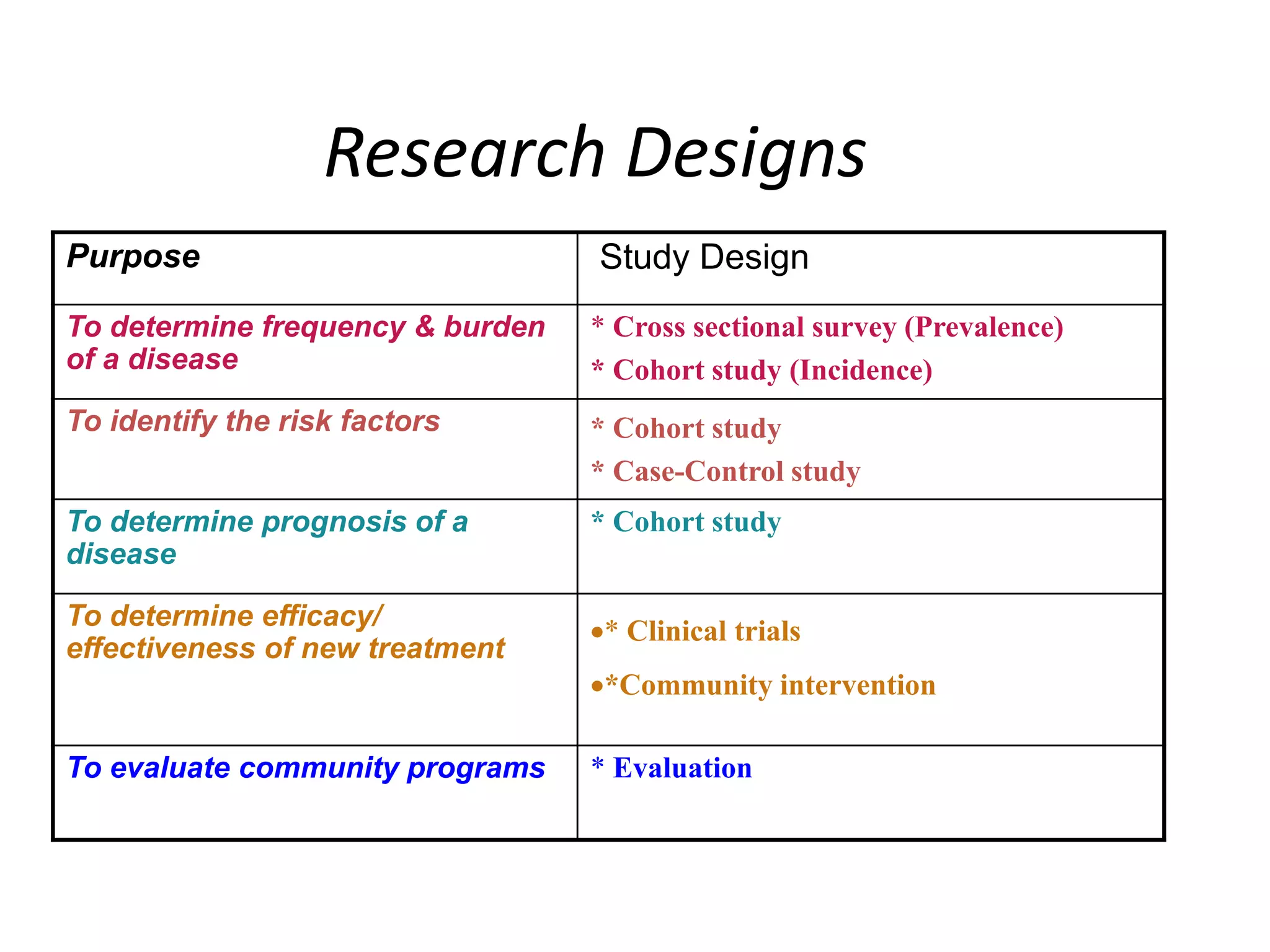
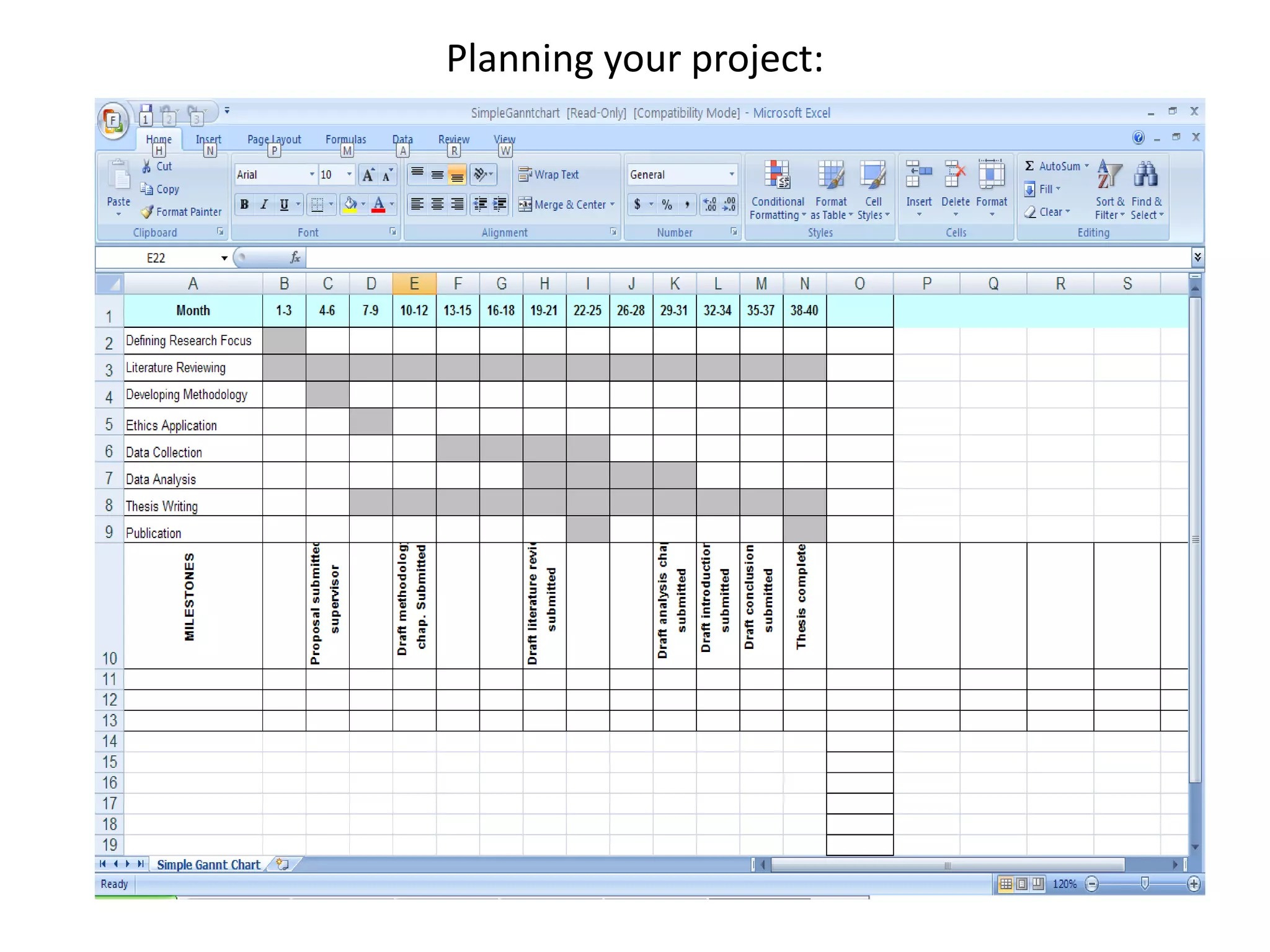
This document provides guidance on developing a research protocol. It explains that a protocol establishes the design and methodology for a research study. The key components of a protocol include the title, introduction/background, objectives, study design, population and recruitment, variables, data collection tools, analysis plan, timeline, and dissemination strategy. Developing a clear protocol is important to gain approval, plan the study, avoid mistakes, and evaluate the research. It should provide enough detail that the study could be replicated by others.