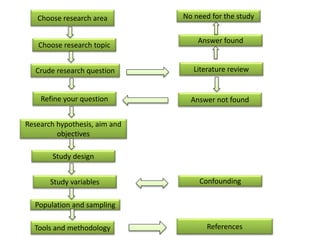
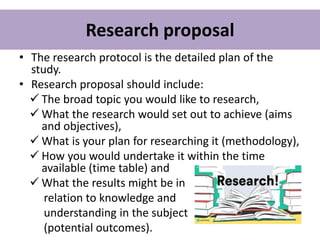
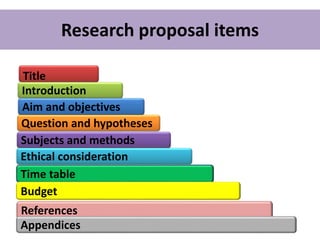
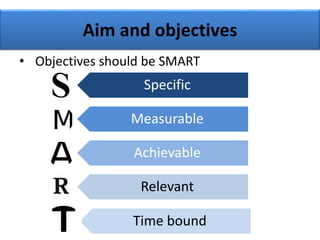
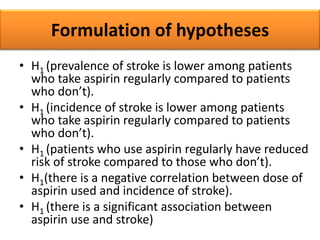
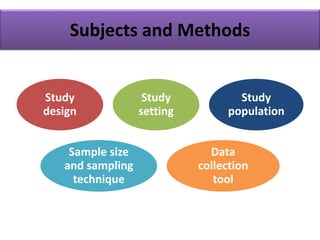
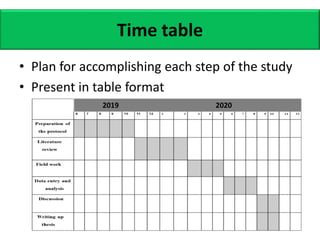
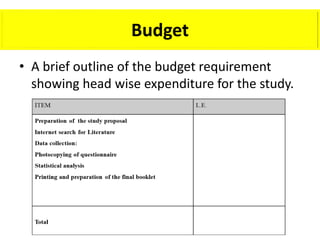
This document provides guidance on how to write a research proposal. It outlines the key elements that should be included such as an introduction defining the research topic and question, objectives, study design, population, methodology, ethical considerations, timeline and budget. A good research proposal convinces others that the proposed study is worthwhile and that the investigator is competent to complete it. Including all relevant components helps ensure the scientific rigor of the planned research.