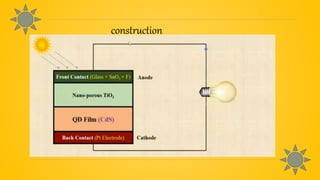
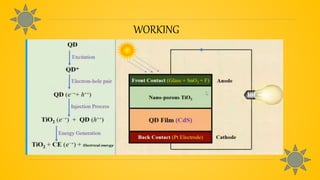
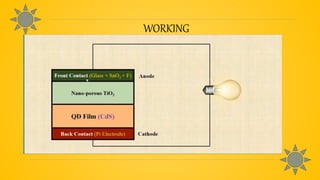
Quantum dot solar cells (QDSCs) utilize tiny semiconductor particles called quantum dots to enhance solar energy absorption and conversion efficiency. They offer advantages such as reduced costs, high efficiency, and the ability to absorb a broad range of the solar spectrum, making them a promising alternative to traditional solar cells. QDSCs work by exciting quantum dots with photons, forming electron-hole pairs, and generating electricity through a series of energy transfer processes.