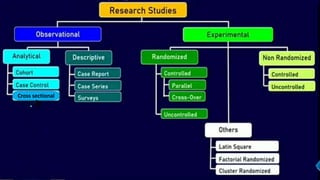
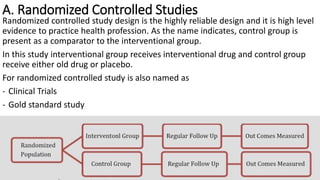
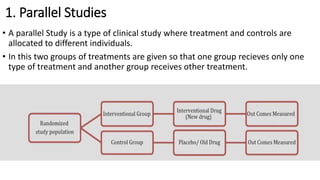
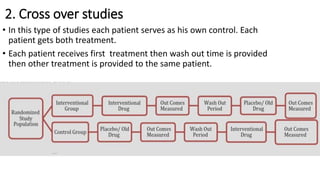
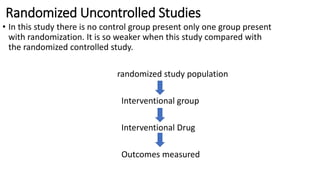
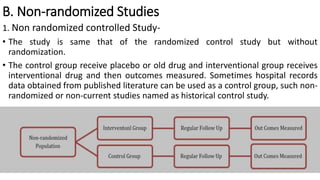
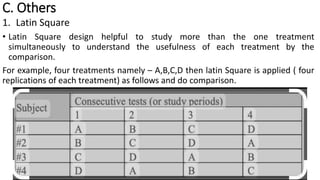
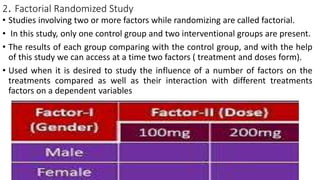
Clinical trials involve experimental studies performed on people to evaluate medical or behavioral interventions. There are various phases of clinical trials, from initial microdosing and Phase I safety studies involving a small number of participants, to larger Phase II and III studies evaluating effectiveness. Phase IV studies monitor safety and additional uses after approval. Experimental studies can be randomized controlled trials, which are the gold standard, or non-randomized designs. Randomized controlled trials involve a treatment and control group, while other designs may lack a control or randomization.










![References -
1.Berrnard Begaud. Dictionary of epidemiology. Published by John Wiley & Sons Ltd, UK,
2000. 7 inventi Rapid: Clinical Research Vol. 2015, Issue 3 [ISSN 0976-383X] 2015 pcr 16325 ©
Inventi Journals (P) Ltd. Published on Web 19/06/2015, www.inventi.in REVIEW ARTICLE
2. G Parthasarathi, Karin Nyfort Hansen, Milap Nahata. A text book of clinical pharmacy.
Published by Universities Press (P) Ltd, Hyderabad, 2013
3. D Pathak. Pharmcoepidemiology: A compement to therapeutic trails, IJPT, 2:1, 2012.
4. R C Goyal. Research methodology for health professionals. Published by Jaypee Brothers
medical publishers (P) Ltd, New Delhi, India, 2013.
5. Ranjan Das, P N Das. Biomedical research methodology. Published by Jaypee Brothers
medical publishers (P) Ltd, New Delhi, India; 2011.
6. Verhamme K M C, Sturkenboom M C J M. Study designs in Paediatric
Pharmacoepidemiology. https://hal.archives-ouvertes.fr/hal-00644718/document.
7. Hilary Anne Wynne, Julia Blagburn. Drug treatment in an ageing population: practical
issue implications, Maturitas, 66:246 – 250, 2010. : falling into a rut. IJP, 35:137-138, 2003.
8.Bernd Rohrig, Jean-Baptist Du Prel, Maria Blettner. Study design in medical research part 2
of a Series on the Evaluation of Scientific Publications. Dtsch Arztebl Int, 106(11): 184–9,
2009.](https://image.slidesharecdn.com/presentation1-1-230323130438-eb5feb90/85/Presentation-1-1-pptx-19-320.jpg)


