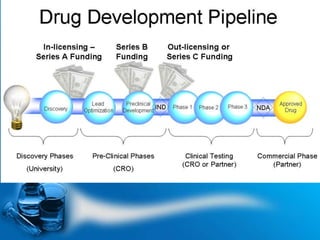
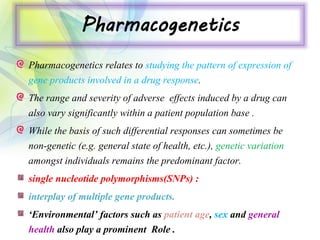
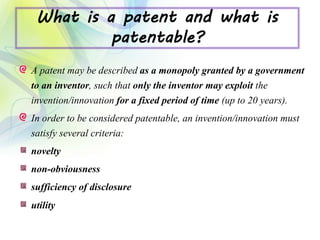
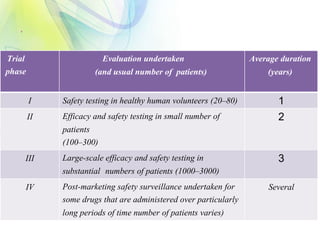
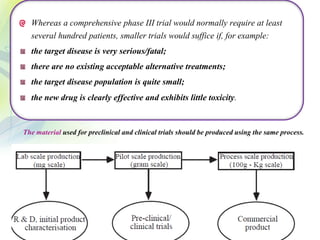
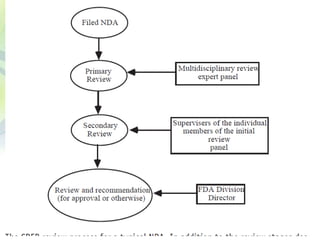
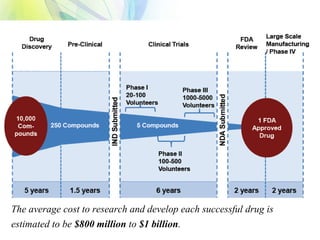
The drug development process is a complex journey that begins with identifying specific diseases and biological targets, followed by drug discovery, optimization, and rigorous testing. Preclinical studies assess safety and efficacy before moving on to clinical trials, which are conducted in phases to ensure thorough evaluation of new drugs. Ultimately, an application for a New Drug Application (NDA) is submitted for regulatory approval, and ongoing monitoring continues post-approval.