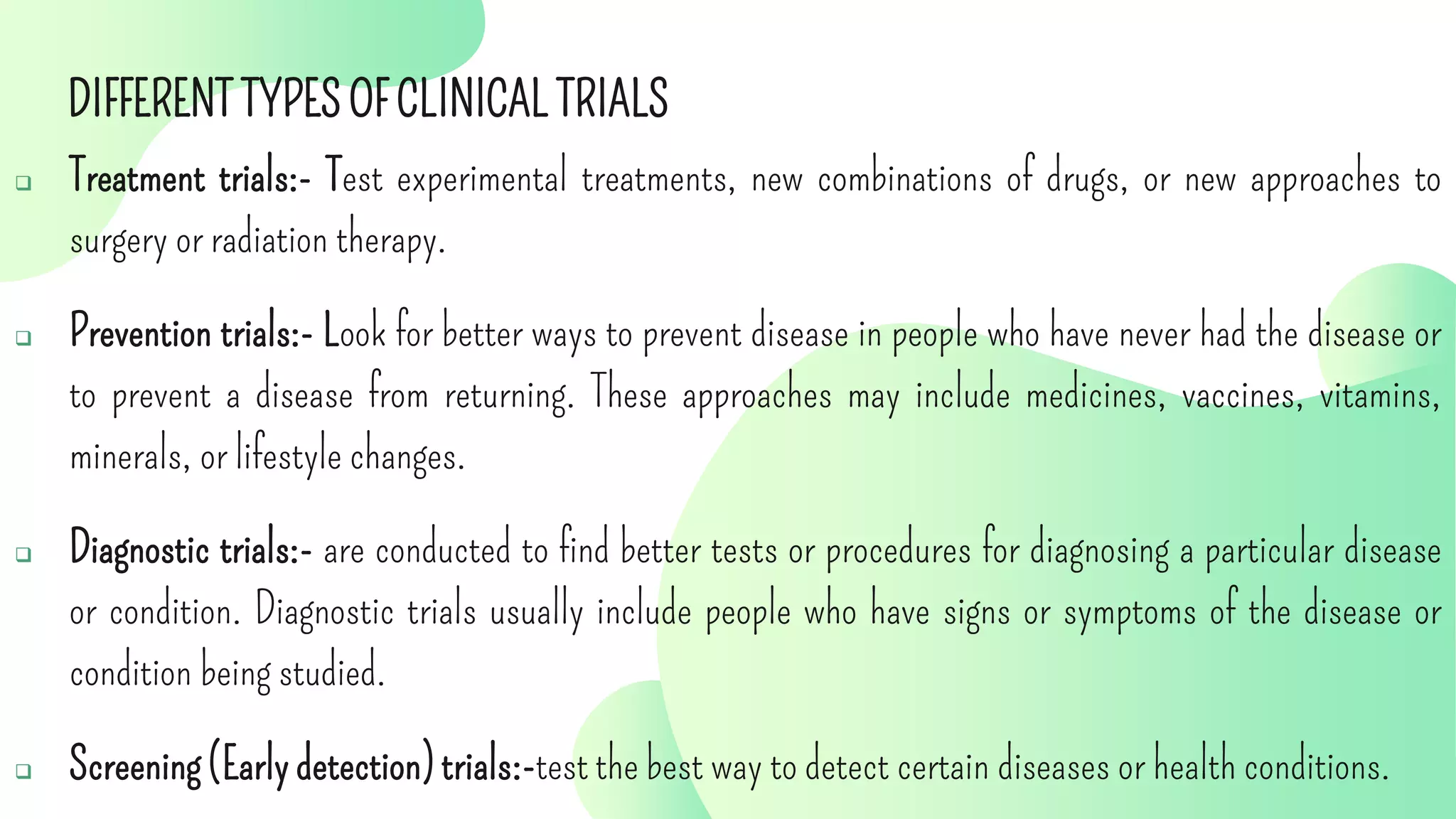
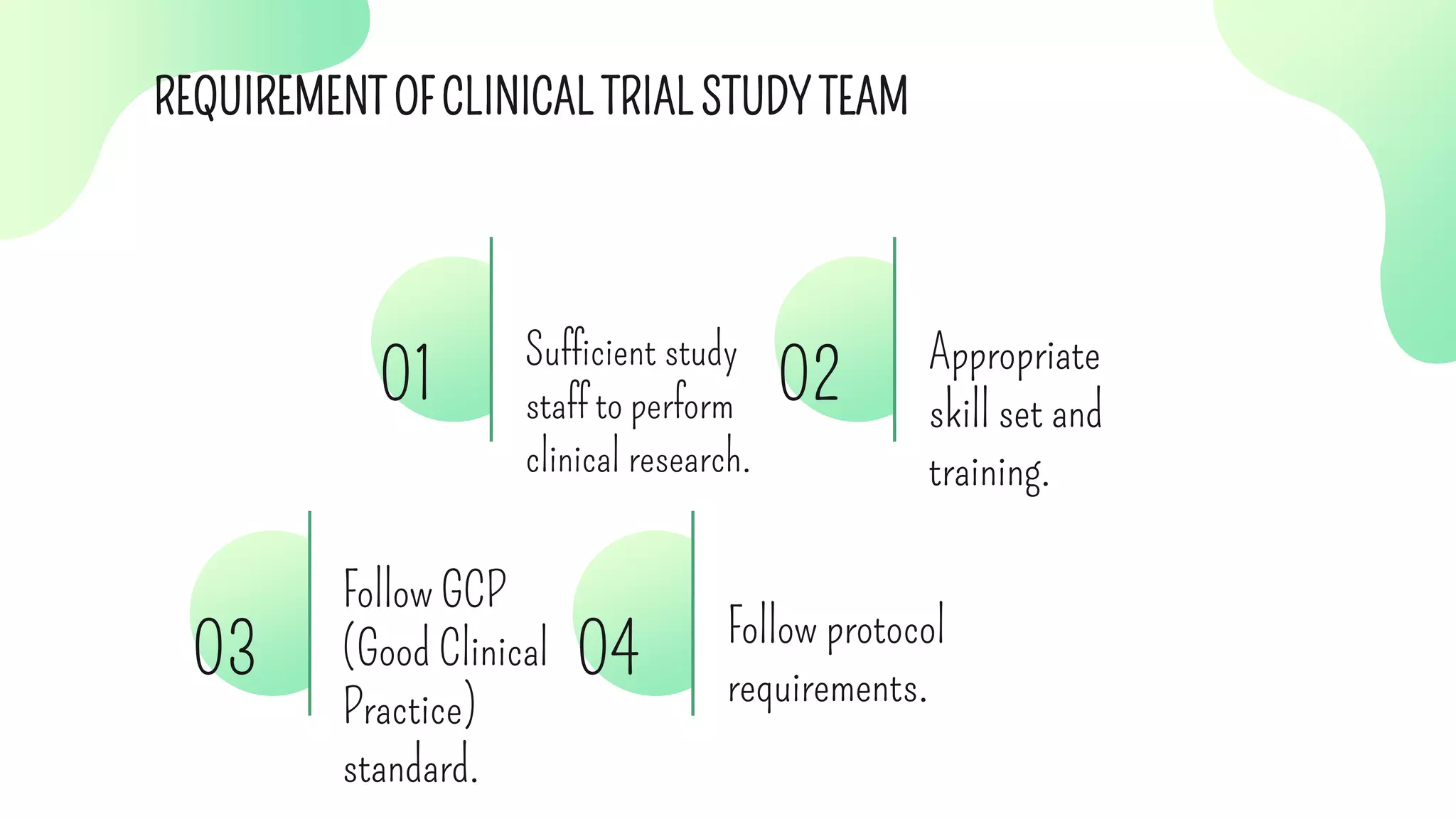
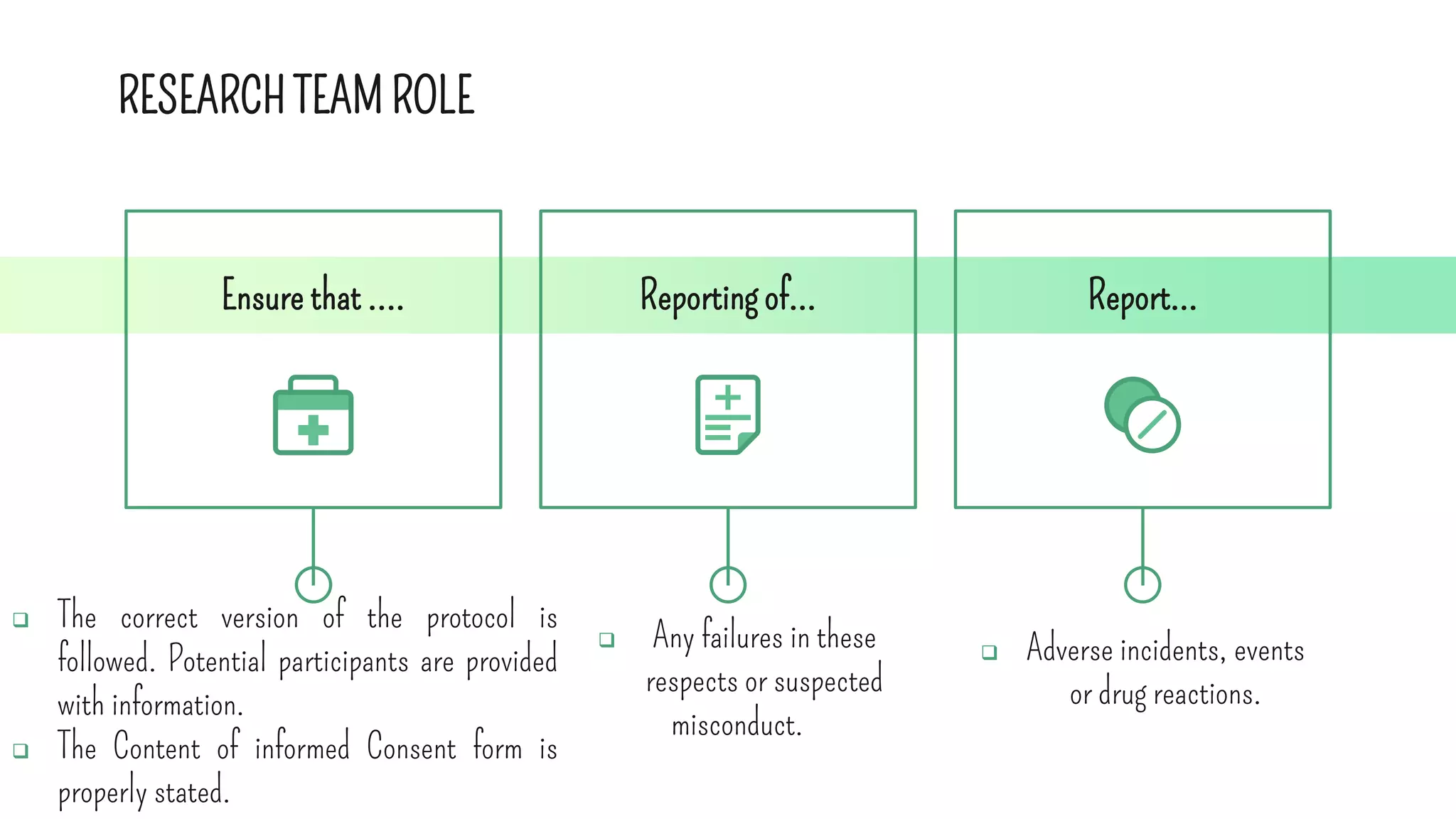
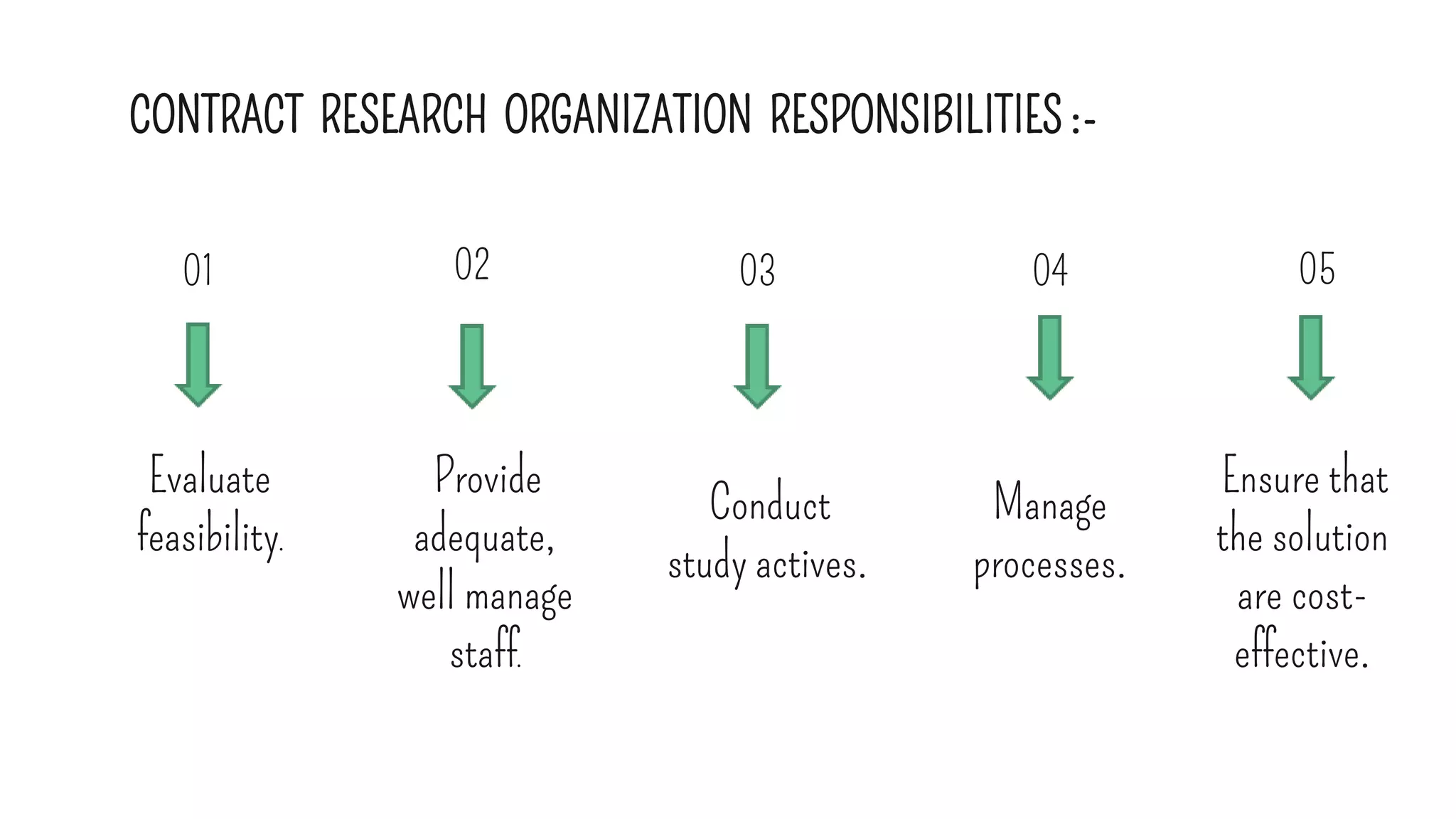
The document outlines the roles and responsibilities of key participants in clinical trials, including investigators, study coordinators, and sponsors, while describing different phases of clinical trials and types of clinical studies. It emphasizes the importance of following good clinical practice (GCP) standards, obtaining informed consent, and ensuring participant safety. Additionally, the document details the functions of contract research organizations in facilitating clinical studies and managing data.