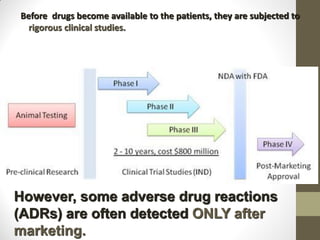
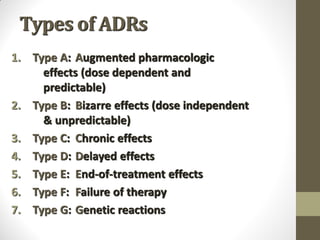
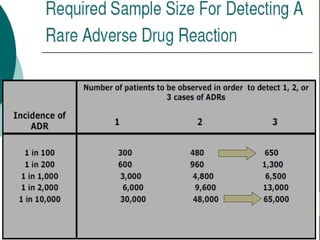
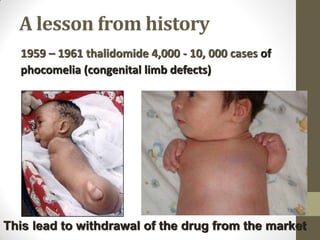
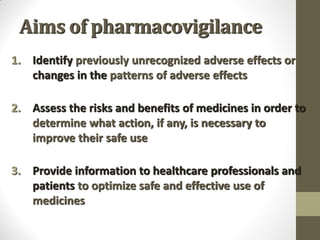
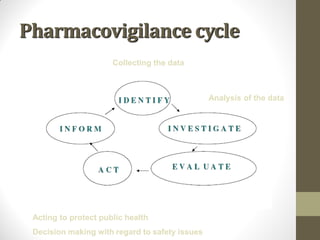
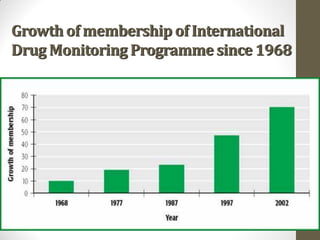
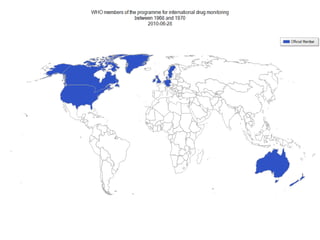
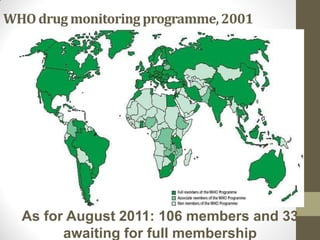
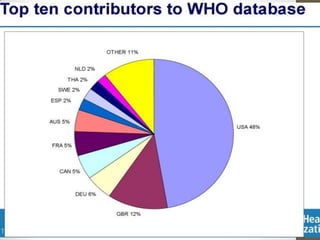
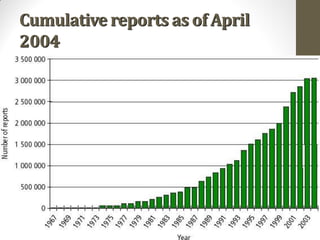
Pharmacovigilance involves monitoring adverse drug reactions (ADRs) that are detected after drugs receive marketing approval. ADRs are defined as harmful effects from normal drug dosages. Several types of ADRs exist and can be serious or severe. Possible causes include drug interactions and underlying medical conditions. Reporting and monitoring ADRs through pharmacovigilance aims to improve patient safety and reduce risks from drug use.