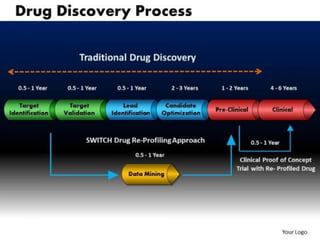
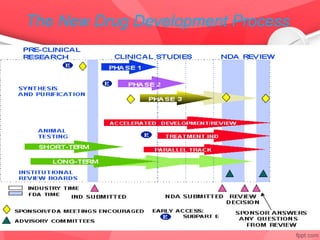
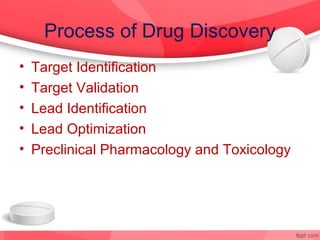
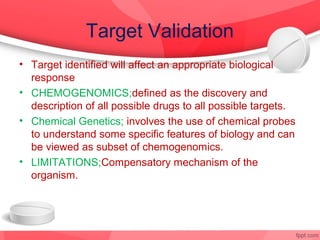
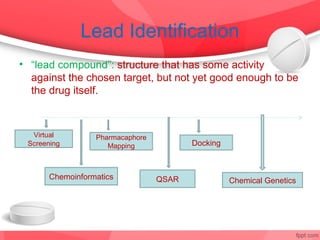
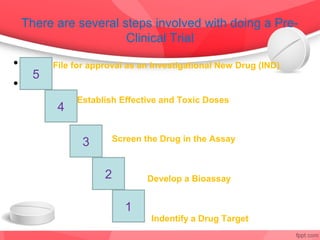
The document provides an overview of the drug discovery process, including the need for new drugs, approaches to discovery, and changes over time. It discusses target identification, validation, lead identification, optimization, and preclinical pharmacology/toxicology. The phases of clinical trials are also summarized, including Phase I safety trials in healthy volunteers, Phase II therapeutic exploration trials, and large Phase III randomized controlled trials. The roles of various parties in clinical trials are also outlined.














![Phase I
• Objectives
1. To assess a safe & tolerated dose
2. To see if pharmacokinetics differ much from animal to man
3. To see if kinetics show proper absorption, bioavailability
4. To detect effects unrelated to the expected action
5. To detect any predictable toxicity
– Inclusion criteria
– Healthy volunteers : Uniformity of subjects: age, sex,
nutritional status [Informed consent a must]
– Exception: Patients only for toxic drugs Eg AntiHIV, Anticancer
– Exclusion criteria
– Women of child bearing age, children,](https://image.slidesharecdn.com/drugdiscovery-121128111904-phpapp02/85/Drug-discovery-By-Neelima-Sharma-WCC-chennai-neelima-sharma60-gmail-com-23-320.jpg)
![Phase II
• First in patient [ different from healthy volunteer]
• Early phase [20 – 200 patients with relevant disease]
– Therapeutic benefits & ADRs evaluated
– Establish a dose range to be used in late phase
– Single blind [Only patient knows] comparison with standard drug
• Late phase [ 50 – 500]
– Double blind
– Compared with a placebo or standard drug
• Outcomes
– Assesses efficacy against a defined therapeutic endpoint
– Detailed P.kinetic & P.dynamic data
– Establishes a dose & a dosage form for future trials
• Takes 6 months to 2 years [ 35% success rate](https://image.slidesharecdn.com/drugdiscovery-121128111904-phpapp02/85/Drug-discovery-By-Neelima-Sharma-WCC-chennai-neelima-sharma60-gmail-com-24-320.jpg)

![Phase IV or Post marketing
Surveillance
• No fixed duration / patient population
• Starts immediately after marketing
• Report all ADRs
• Helps to detect
– rare ADRs
– Drug interactions
– Also new uses for drugs [Sometimes called Phase V]](https://image.slidesharecdn.com/drugdiscovery-121128111904-phpapp02/85/Drug-discovery-By-Neelima-Sharma-WCC-chennai-neelima-sharma60-gmail-com-26-320.jpg)

![Participating Parties in Clinical Trial
1. Patient / Healthy volunteer: Subject of the trial
2. Clinical Pharmacologist, Clinical Investigator & team: [Qualified and
competent] Conducts the clinical trial; reports all adverse events
3. Institution where trials are held : [Approval required] Provides all
facilities
4. Ethical Review Board or Institutional Ethical Committee:
-Supervises and monitors every step;
– Safeguard the welfare and the rights of the participants
– 5. Sponsor
– Pays for all expenses;
– Appoints competent investigators,
– Ships all drugs for the trial,
– Files all papers to legal / regulatory authorities,
6. Regulatory Authorities:
Legal authority on the outcomes of the trial](https://image.slidesharecdn.com/drugdiscovery-121128111904-phpapp02/85/Drug-discovery-By-Neelima-Sharma-WCC-chennai-neelima-sharma60-gmail-com-28-320.jpg)
