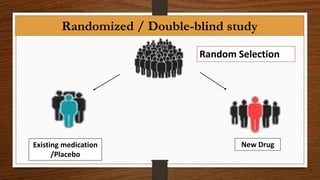
The document summarizes the key steps in the drug discovery and development process. It begins with target identification and validation to find potential drug targets. High throughput screening is then used to identify initial hit compounds. Hits undergo further testing including assays and screening to identify lead compounds. Lead optimization is used to improve potency and safety. If leads are promising, they undergo pre-clinical animal testing before human clinical trials. Clinical trials involve 4 phases to test safety, efficacy, and dosing in humans. If successful, the drug sponsor submits a New Drug Application to the FDA for review and potential approval.