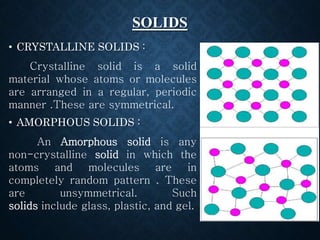
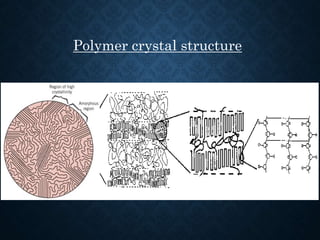
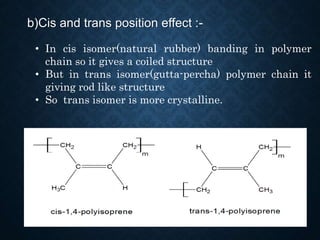
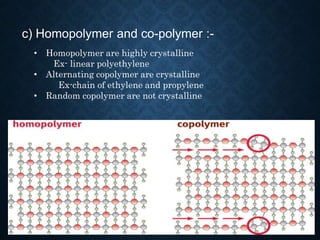
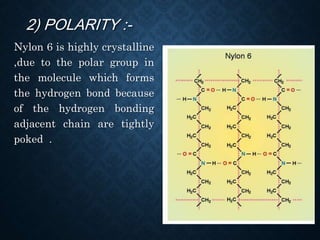
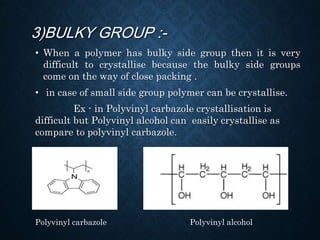
This document discusses crystallinity and factors that affect crystallizability in polymers. It defines crystalline and amorphous solids, and explains that polymers exist as both crystalline and amorphous regions. Crystallinity refers to the amount of crystalline regions relative to amorphous regions. Crystallizability is the maximum crystallinity achievable at a given temperature. Factors that affect crystallizability include the polymer's molecular structure, molecular weight, branching, cis/trans configuration, homo- or co-polymer composition, polarity, and presence of bulky side groups. Homopolymers with linear and alternating structures tend to be more crystalline, while randomness, branching, and bulky groups inhibit crystallization.