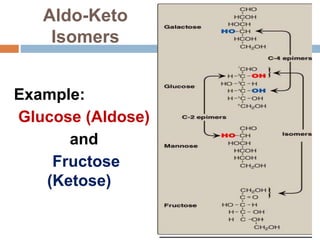
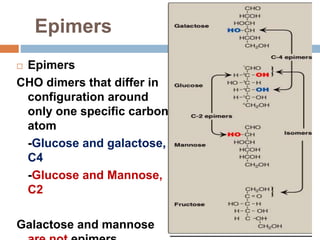
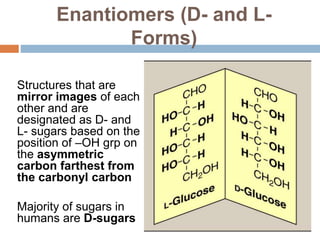
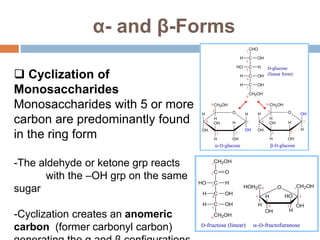
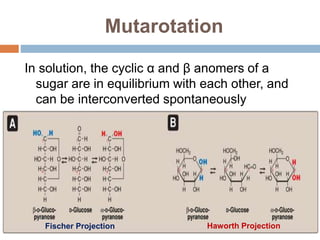
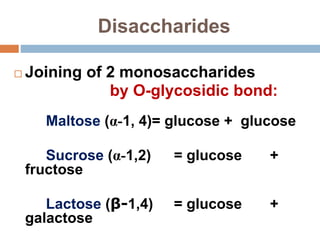
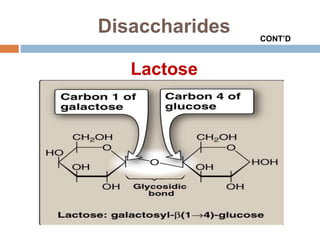
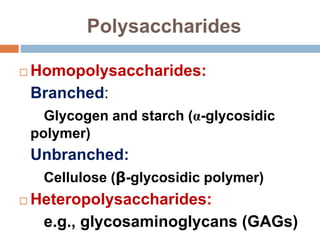
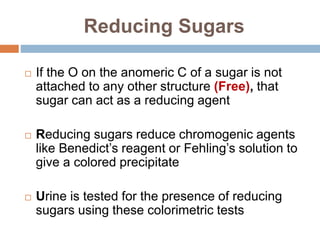
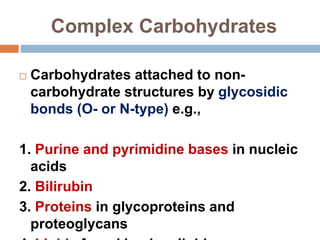
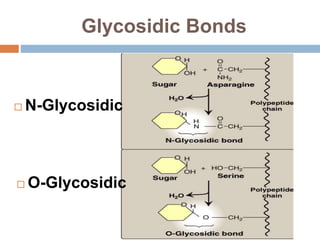
The document discusses the structure and function of carbohydrates, emphasizing their role in energy provision, storage, and cellular structure. It details various carbohydrate classifications, including monosaccharides, disaccharides, and polysaccharides, alongside their isomers and glycosidic bonds. Additionally, it highlights the significance of glycosaminoglycans (GAGs) in biological systems, including their properties and functions.





![Glycosaminoglycans (GAGs)
GAGs are linear polymers of repeating
disaccharide units
[acidic sugar-amino sugar]n
The amino sugar (usually sulfated) is
either
D-glucosamine or D-
galactosamine
The acidic sugar is either
D-glucuronic acid or L-iduronic
acid
GAGs are strongly negatively-charged:](https://image.slidesharecdn.com/lecture4-structureandfunctionofcarbohydrates-240421104913-966ec20f/85/Lecture-4-Structure-and-function-of-carbohydrates-ppt-23-320.jpg)